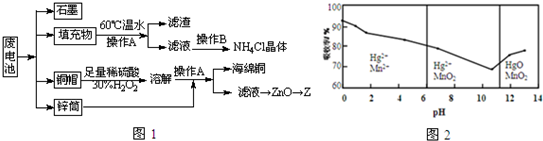
��Ŀ����
8��ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�ݡ������е������Լ����գ����ȶ�����ģ���֪�������ԣ�IO3-��Fe3+��I2����ԭ�ԣ�S2O32-��I-3I2+6OH-�T5I-+IO3-+3H2O KI+I2?KI3
��1��ij��ȤС��Լӵ��ν���������ʵ�飺ȡһ����ij�ӵ��Σ����ܺ���KIO3��KI��Mg2+��Fe3+��������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݣ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ��������Һ�м�������KIO3����μӵ����Լ�������������
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ���������軯����д�����ƣ���CCl4�����Ϻ�ɫ��������
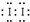 ���õ���ʽ��ʾ����
���õ���ʽ��ʾ�����ڵڶ�����Һ�м�������KI��������ȷ�����Ӧ��Ӧ�����ӷ���ʽΪIO3-+5I-+6H+�T3I2+3H2O��
��2��KI��Ϊ�ӵ��ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ4KI+O2+2H2O�T2I2+4KOH��
��3���Ժ�Fe2+�϶��ʳ�Σ����費��Fe3+������ѡ��KI��Ϊ�ӵ���������ʵ�鷽��������üӵ����е�Fe2+��ȡ�����üӵ�����������ˮ�У��������ữ���μ��������������磺��ˮ����������ȣ����ٵμ�KSCN��Һ������Ѫ��ɫ����üӵ����д���Fe2+��ֻ������Լ������ƣ�
���� ��1���ٸ����������У�ֻ��Fe3+��KSCN��Һ�Ժ�ɫ��Fe3+��KI����������ԭ��Ӧ���ɵĵⵥ�ʣ��������л��ܼ����ҵ������Ȼ�̼������ɫ��
����Ϊ�����ԣ�IO3-��Fe3+��I2�����Եڶ�����Һ�м�������KI��������ȷ�����Ӧ��Ӧ�����ӷ���ʽΪIO3-+5I-+6H+�T3I2+3H2O��
��2������KI���л�ԭ�Լ�������ԭ��Ӧ��������
��3������������ת��Ϊ�����ӣ���������������KSCN��Һ���ɫ�����飮
��� �⣺��1��ij�ӵ��ο��ܺ���KIO3��KI��Mg2+��Fe3+��������ˮ�ܽ⣬����ϡ�����ữ����Һ��Ϊ3�ݣ��ӵ�һ����Һ�еμ�KSCN��Һ���Ժ�ɫ����֪�üӵ����к���Fe3+����Ӧ��Fe3++3SCN-=Fe��SCN��3��Fe��SCN��3��Ѫ��ɫ���ӵڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����֪�е����ɣ�������Ϊ���ڡ������ԣ�IO3-��Fe3+��I2����������KI��IO3-��Fe3+���ܽ�I-������I2���ɴ�Ҳ����֪���üӵ�������KIO3����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ���ɴ˿�֪�üӵ����в���KI��
�ٸüӵ�����Һ�м�KSCN��Һ�Ժ�ɫ��������Fe��SCN��3������Ϊ�����軯����CCl4�����Ϻ�ɫ��������I2������ʽ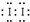 ���ʴ�Ϊ�����軯����
���ʴ�Ϊ�����軯����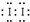 ��
��
����Ϊ�����ԣ�IO3-��Fe3+��I2�����Եڶ�����Һ�м�������KI��������ȷ�����Ӧ��Ӧ�����ӷ���ʽΪIO3-+5I-+6H+�T3I2+3H2O���ʴ�Ϊ��IO3-+5I-+6H+�T3I2+3H2O��
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У�KI�ᱻ����������������KI�ڳ�ʪ�����������ķ�Ӧ��ѧ����ʽΪ��4KI+O2+2H2O�T2I2+4KOH���ʴ�Ϊ��4KI+O2+2H2O�T2I2+4KOH��
��3��ʵ���Ͼ������ʵ�鷽��������Fe2+�����ȿ�ȡ�����üӵ�����������ˮ�У�Ȼ���������ữ�μ��������������磺��ˮ����������ȣ���ʹ��Һ��Fe2+ת��ΪFe3+���ٵμ�KSCN��Һ������Ѫ��ɫ����üӵ����д���Fe2+���ʴ�Ϊ��ȡ�����üӵ�����������ˮ�У��������ữ���μ��������������磺��ˮ����������ȣ����ٵμ�KSCN��Һ������Ѫ��ɫ����üӵ����д���Fe2+��
���� �������ճ���������Ϥ��ʳ�κͼӵ����е����ʧԭ��Ϊ�زģ�Ҫ��ͨ��ʵ�飬����ӵ����������еijɷ����ʣ�̽��������������ڿ��������������������������ʧ�ķ�Ӧ��̽��KI3•H2O��Ϊʳ�μӵ���Ƿ���ʡ��Լ���ӵ��Σ�����KI�����ȶ��Կ����ӵ��ȶ��������ʵ�鷽������ѡ��KI��Ϊ�ӵ���ļӵ����е�Fe2+�ȣ��Ӷ��������ʵļ��顢������ԭ��Ӧ��������ԭ��Ӧ����ʽ�����ӷ�Ӧ�����ӷ�Ӧ����ʽ������ʽ���Լ�ʵ�鷽������ƵȻ�ѧ����֪ʶ�ͻ������ܣ�����ͻ����Ч��ȡ֪ʶ��������֪ʶ���ϵ�������ͻ�����ܹ�������Ϣ��ȷ��ȡʵ���Ե����ݣ�������ѧ��֪ʶ������Ч��ϣ����ʵ��������������������¿γ̡�����˵��������ѧ���֣���ѧϰ������Ҫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | Aһ��������̬���� | B�� | Aһ������̬���� | ||
| C�� | Bһ��������̬���� | D�� | Bһ������̬���� |
C2H4��g��+H2O��g��=C2H5OH��g����H=-45.8kJ/mol
������˵������ȷ���ǣ�������
| A�� | ʵ���У���ϩ����������Ӱ��÷�Ӧ�ķ�Ӧ�ʱ��H | |
| B�� | 0.5 mol H2O��l�� ��ȫ��Ӧ�ų�������Ϊ22.9 kJ | |
| C�� | 1 mol C2H5OH��g�������������1 mol C2H4��g����1 mol H2O ��g������������� | |
| D�� | 1 mol C2H4��g����1 mol H2O ��g���л�ѧ�����ܼ��ܴ���1 mol C2H5OH��g���л�ѧ�����ܼ��� |
| A�� | 2LCO��2L CO2 | |
| B�� | 9��H2O�ͱ�״����11.2L CO2 | |
| C�� | ��״����1mol O2��22.4L H2O | |
| D�� | 0.2mol H2�ͱ�״����4.48L HCl���� |
| A�� | 0.1mol�����Ӻ��е�����Ϊ1.1NA | |
| B�� | �ڳ��³�ѹ�£�1mol������е�ԭ����Ϊ2NA | |
| C�� | 1molNa2O2�к���������Ϊ3NA | |
| D�� | 1molCCl2F2�к�������������ΪNA |