��Ŀ����
16����ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ģ��������ʵ�鳣�õ��������ش����⣺
��1����д�������������ƣ�B��ƿD��Һ©��E������ƿGţ�ǹܣ�
��2�������Ȼ�̼��ȡ����ˮ��Br2���ӵķ�����������ȡ����Br2�����Ȼ�̼��Һ��ˮ���뿪�IJ�����������Һ�������ǽ��иò����IJ��裬��ȷ����˳���ǣ��ݢ٢ۢܢڣ�
�ٽ�����ﵹ���Һ©���У�����������̨����Ȧ�Ͼ��ã��ֲ㣻
�ڴӷ�Һ©���Ͽڵ����ϲ���Һ
�۽���Һ©���IJ�������ʹ�������ϵİ��۶�©��������С��
�ܷ�Һ©�����浼�ܽ����ձ��ڱڣ��������������ձ�������Һ
�ݼ���Һ©�������;����IJ������Ƿ�©ˮ
��3����֪Br2�ķе���58.5��C�����Ȼ�̼�е���78��C����Br2�����Ȼ�̼��Һ���뿪�ķ�����������Ҫ�õ��IJ��������У�����ĸ��BEGHJK��
���� ��1�����������Ĺ����Լ���״���ж����ƣ�
��2�������������Ȼ�̼��������ȡ�ķ������룻���÷�Һ�ķ����ɷ��뻥�����ܵ�Һ�壬��ҺʱӦ�ȼ���Ƿ�©ˮ��������ﵹ���Һ©���У�����������̨����Ȧ�Ͼ��á��ֲ㣬����Һ©���IJ�������ʹ�������ϵİ��۶�©��������С�ף���Һ©�����浼�ܽ����ձ��ڱڣ��������������ձ�������Һ���ӷ�Һ©���Ͽڵ����ϲ�ˮ��Һ��
��3����������ķ�������е㲻ͬ��Һ�����
��� �⣺��1����������ͼ�ο�֪��BΪ��ƿ��DΪ��Һ©����EΪ������ƿ��GΪţ�ǹܣ�
�ʴ�Ϊ����ƿ����Һ©����������ƿ��ţ�ǹܣ�
��2�������������Ȼ�̼��������ȡ�ķ������룬���÷�Һ�ķ����ɷ��뻥�����ܵ�Һ�壬��ҺʱӦ�ȼ���Ƿ�©ˮ��������ﵹ���Һ©���У�����������̨����Ȧ�Ͼ��á��ֲ㣬����Һ©���IJ�������ʹ�������ϵİ��۶�©��������С�ף���Һ©�����浼�ܽ����ձ��ڱڣ��������������ձ�������Һ���ӷ�Һ©���Ͽڵ����ϲ�ˮ��Һ��˳��Ϊ�ݢ٢ۢܢڣ�
�ʴ�Ϊ����ȡ����Һ���ݢ٢ۢܢڣ�
��3����������ķ�������е㲻ͬ��Һ�������Ҫ�õ���������BEGHJK��
�ʴ�Ϊ������BEGHJK��
���� �����ۺϿ������ʵķ��롢�ᴿ�Լ����������ʹ�õ�֪ʶ��Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע�����ʵ��IJ����������ѶȲ���

| A�� | CaO+H2O�TCa��OH��2 | B�� | H2+Cl2�THCl | C�� | Na2O+H2O�T2NaOH | D�� | NH3+HCl�TNH4Cl |
| A�� | ����Ũ����մ��Ƥ���ϣ�����������������Һ��ϴ | |
| B�� | ��ȡ���ռ�����������Ӧ����ֹͣ���� | |
| C�� | ������CO�ж�ʱ��Ӧ�������ж���̧���������ʿ����� | |
| D�� | ��������ķ�Һ����ˮ�ۣ���ˮ������ˮ�� |
| A�� | Cԭ�ӵĹ���ӻ����ͷֱ�Ϊsp��sp2��sp3 | |
| B�� | �������C2H6��C2H4��C2H2 | |
| C�� | �Ҽ�������C2H6��C2H4��C2H2 | |
| D�� | ̼̼����ļ��ܣ�C2H6��C2H4��C2H2 |
| ���� | 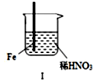 |  | |
| ���� | Fe�������������ɫ���ݣ�Һ���Ϸ���Ϊ����ɫ | Fe���������������ɫ���ݺ�Ѹ��ֹͣ | Fe Cu�Ӵ�����������������ɫ���� |
| A�� | ������������ɫ�����ɫ�Ļ�ѧ����ʽΪ��2NO+O2=2NO2 | |
| B�� | �ԱȢ�������˵��ϡHNO3��������ǿ��ŨHNO3 | |
| C�� | ���е�����˵��Fe�����γ����ܵ������㣬��ֹFe��һ����Ӧ | |
| D�� | ��Ԣ���������Fe��Cu֮�����ӵ����ƣ����ж�Fe�Ƿ����� |
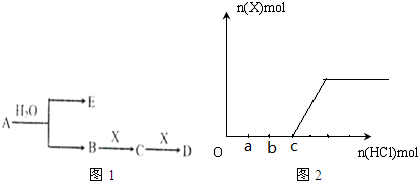
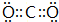
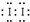 ���õ���ʽ��ʾ����
���õ���ʽ��ʾ����