��Ŀ����
18��������ԭ��Ӧ��ʵ���ϰ��������ͻ�ԭ�������̣�������һ����ԭ���̵ķ�Ӧʽ��NO3-+4H++3e-=NO+2H2OKMnO4��Na2CO3��Cu2O��Fe2��SO4��3���������е�һ�����ʣ��ף���ʹ������ԭ���̷�����
��1��д������ƽ��������ԭ��Ӧ�ķ���ʽ��14HNO3+3Cu2O=6Cu��NO3��2+2NO��+7H2O
��2����Ӧ���������������ԡ����������ʣ�
��3����Ӧ��������0.2mol���壬��ת�Ƶ��ӵ����ʵ�����0.6mol��
��4����1mol����ijŨ�����ᷴӦʱ������ԭ��������ʵ������ӣ�ԭ���ǣ�ʹ���˽�Ũ�����ᣬ�������в��ֶ�����������
��5��KMnO4��������Һ�з�����ԭ��Ӧ�ķ�Ӧʽ��MnO4-+8H++5e-=Mn2++4H2O��
���� ��1��������������ԣ���Ŀ�ṩ������������ֻ��Cu2O���л�ԭ�ԣ����Է�Ӧ�ķ���ʽΪ������Cu2O��Ӧ��������ͭ��NO��ˮ����ƽ��д����ʽ��
��2�����ݵ�Ԫ�صĻ��ϼ��жϷ�Ӧ��������������ã�
��3����Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+5�۽���Ϊ+2�ۣ����ݵ�ʧ�����غ��֪��ת�Ƶ������ʵ�����NO���ʵ�����3����
��4��ʹ���˽�Ũ�����ᣬ�������в��ֶ����������ɣ������ᱻ��ԭΪNO2ʱ����λ������Cu2O���ĵ����������ӣ�
��5��������������������·�����ԭ��Ӧ����������
��� �⣺��1��������������ԣ���Ŀ�ṩ������������ֻ��Cu2O���л�ԭ�ԣ����Է�Ӧ�ķ���ʽΪ������Cu2O��Ӧ��������ͭ��NO��ˮ����Ӧ����ʽΪ��14HNO3+3Cu2O=6Cu��NO3��2+2NO��+7H2O��
�ʴ�Ϊ��14HNO3+3Cu2O=6Cu��NO3��2+2NO��+7H2O��
��2����Ӧ�������е�Ԫ�ش�������ͭ��NO�У�����ͭ�е�Ԫ�ػ��ϼ۲��䣬����������ԣ�NO�е�Ԫ�ػ��ϼ۽��ͣ�������������ԣ�
�ʴ�Ϊ�����ԡ������ԣ�
��3����Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+5�۽���Ϊ+2�ۣ�ת�Ƶ������ʵ���Ϊ0.2mol����5-2��=0.6mol��
�ʴ�Ϊ��0.6��
��4��ʹ���˽�Ũ�����ᣬ�������в��ֶ����������ɣ������ᱻ��ԭΪNO2ʱ����λ������Cu2O���ĵ����������ӣ�
�ʴ�Ϊ��ʹ���˽�Ũ�����ᣬ�������в��ֶ����������ɣ�
��5��������������������·�����ԭ��Ӧ���������ӣ�ͬʱ����ˮ�����ӷ���ʽΪMnO4-+8H++5e-=Mn2++4H2O���ʴ�Ϊ��MnO4-+8H++5e-=Mn2++4H2O��
���� ���⿼��������ԭ��Ӧ������д����ƽ����������йؼ���ȣ��ѶȲ���4��Ϊ�״��㣬ע�����Ũ���ᡢϡ����Ļ�ԭ���ﲻͬ���н��

| A�� | ����ӵ�غͼ���п�̸ɵ�ض��Ƕ��ε�� | |
| B�� | ȼ�ϵ����һ�ָ�Ч���ǻ���Ⱦ���������͵�� | |
| C�� | ��ѧ��صķ�Ӧ������������ԭ��Ӧ | |
| D�� | Ǧ���طŵ��ʱ��������Pb��������PbO2 |
 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�������Ͳ��ȡ50mL 0.5mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL 0.6mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȣ�
�ش��������⣺
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ���к�����ֵΪ57.3kJ/mol����HCl ��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3 kJ/mol��
��2������NaOH��Һ����ȷ������C��������ѡ������
A���ز������������롡B���������������� C��һ��Ѹ�ٵ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������D��������ѡ������
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
��4��ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ | ||
| HCl | NaOH | ƽ��ֵ | ��t2-t1��/�� | ||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��abcd��
a��ʵ��װ�ñ��¡�����Ч����
b��������ת����С�ձ�ʱ����һ����������
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ���¶ȣ�
| A�� | �����Ũ�� | B�� | ��Һ���¶� | C�� | Zn�����Ĵ�С | D�� | ��Һ��Cl-��Ũ�� |
| A�� | XW4Ϊ�Ǽ��Է��� | |
| B�� | Y��Z��W������������ˮ��������ǿ��˳����Y��Z��W | |
| C�� | X��Y������������ɹ�̬ת��Ϊ��̬ʱ���˷���ͬ�������� | |
| D�� | XW4��YW4��ZW2�����е�����ԭ�Ӿ�Ϊsp3�ӻ� |
| A�� | KHCO3��MgCO3 | B�� | MgCO3��NaNO3 | C�� | BaCO3��NaHCO3 | D�� | Na2CO3��NaHCO3 |
| A�� | ������SO2ͨ�백ˮ�У�SO2+2NH3•H2O�T2NH4++SO32-+H2O | |
| B�� | SO2ͨ����ˮ�У�Br2+SO2+2H2O�T2H++SO42-+2HBr | |
| C�� | ����SO2ͨ��Ư����Һ�У�SO2+H2O+Ca2++2ClO-�TCaSO3��+2HClO | |
| D�� | �������pH����2H2SO3+O2�T4H++2SO42- |

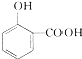 ���м��ƷC�л��з�Ӧ��A��B������C�к���A���Լ���NaHCO3��Һ��
���м��ƷC�л��з�Ӧ��A��B������C�к���A���Լ���NaHCO3��Һ��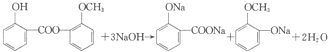 ��
��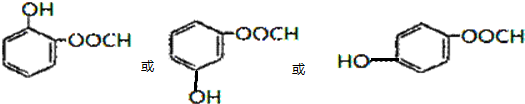 ��
��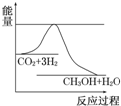 Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ•mol-1���ı仯�������Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��Ӧ��
Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ•mol-1���ı仯�������Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��Ӧ��