��Ŀ����
9����1����ҵ�����������к��д���SO2�����д��������к�������bc������ţ���a���߿��ŷ� b���ð�ˮ�����Ʊ����� c���ô�����Һ������Na2SO3 d����Ũ��������
��2������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬ�õ���ö�ײ������缫�������������壬ͬʱҲ��ʹ������������Һ��ֽӴ����õ�ظ����ĵ缫��ӦΪSO2-2e-+2H2O�TSO42-+4H+��
��3������Na2SO3��Һ�������SO2�Ƶ�NaHSO3��Һ����֪NaHSO3�����ԣ���������Ũ�ȹ�ϵ�ж���ȷ����bd�����ţ���
a��Na2SO3��Һ��c��Na+����c��SO32-����c��HSO3-����c��OH-����c��H+��
b��NaHSO3��Һ��c��SO32-����c��H2SO3��
c�������ʵ�����NaHSO3��Na2SO3����ˮ������Һ�У�3c��Na+��=2c��HSO3-��+2c��H2SO3��
d���ڷ�Ӧ������һ������c��Na+��+c��H+��=c��HSO3-��+2c��SO32-��+c��OH-��
��4������NaHSO3��Һ���Ƶ����ᣬͬʱ����Һ����������ԭ����ͼ��ʾ����Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��������a�����ӵ�Դ�ĸ����������������������ʼʱ�����ĵ缫��ӦʽΪHSO3-+H2O-2e-=SO42-+3H+��
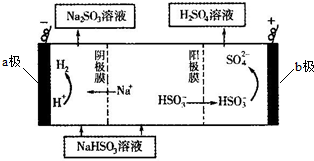
���� ��1��ұ�����̲�������SO2�����������к����ķ����Ƕ�����������Ⱦ�����壬�����ŷŵ������У������������ã�
��2������ԭ���ԭ����������ʧ���ӷ���������Ӧ����������ʧ������������Ĺ��̣�
��3��a��Na2SO3��Һ�У���������������ˮ�ĵ��������������ӵ�ˮ�⣬��c��OH-����c��HSO3-����
b��NaHSO3��Һ��ʾ���ԣ���������������ӵĵ���̶ȴ�����ˮ��̶ȣ���c��SO32-����c��H2SO3����
c�����ݵ����ʵ�����NaHSO3��Na2SO3����ˮ������Һ�е������غ��жϣ�
d�����ݻ��Һ�еĵ���غ������
��4��a�������ӱ��������������ԭ��Ӧ����aΪ���������ӵ��ǵ�Դ�ĸ��������ݻ��ϼ۱仯�ж�������Ӧ���ʣ�д��������Ӧʽ��
��� �⣺��1��a��������������Ⱦ�����壬�߿��ŷŻ���Ⱦ��������������������a����
b����ˮ���������Ӧ����������泥������ð�ˮ�����Ʊ����ʣ���b��ȷ��
c���ô�����Һ���ն������������Na2SO3����c��ȷ��
d����Ũ��������ն�������d����
�ʴ�Ϊ��bc��
��2����ԭ����У�������ʧ���ӱ������������������ᣬ��Ļ��ϼ����ߣ����Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ����Ը����ϵĵ缫��ӦʽΪ��SO2-2e-+2H2O�TSO42-+4H+��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
��3��a��Na2SO3��Һ�У�����������Ӳ���ˮ�⣬��Һ��ʾ���ԣ�������������������ˮ�ĵ��������������ӵ�ˮ�⣬��c��OH-����c��HSO3-������Һ������Ũ�ȴ�СΪ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+������a����
b��NaHSO3��Һ�����ԣ�����HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ���c��SO32-����c��H2SO3������b��ȷ��
c�������ʵ�����NaHSO3��Na2SO3����ˮ������Һ�У����������غ�ɵã�2c��Na+��=3c��HSO3-��+3c��H2SO3��+3c��SO32-������c����
d����Һ��һ���������غ㣬���ݵ���غ�ɵã�c��Na+��+c��H+��=c��HSO3-��+2c��SO32-��+c��OH-������d��ȷ��
�ʴ�Ϊ��bd��
��4������ͼʾ��֪��a�������ӵõ����ӱ��������������ԭ��Ӧ����aΪ������a���ӵ�Ϊ��Դ�ĸ�����
�����������͵�Դ����������ʧȥ���ӣ�����������Ӧ�����NaHSO3��Һ���Ƶ����ᣬ��Ļ��ϼ����ߣ�����������HSO3-��Һʧȥ���ӱ���������SO42-���������缫��Ӧʽ�ǣ�HSO3-+H2O-2e-=SO42-+3H+��
�ʴ�Ϊ��������HSO3-+H2O-2e-=SO42-+3H+��
���� ���⿼��������Ũ�ȴ�С�Ƚϡ����ԭ����ԭ���ԭ������Ӧ�õ�֪ʶ����Ŀ�Ѷ��еȣ�ע�����յ��ԭ����ԭ���ԭ������ȷ����غ㡢�����غ㼰�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�ã�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | 2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O | B�� | H2��2H | ||
| C�� | CaO+H2O=Ca��OH��2 | D�� | 2Cl��Cl2 |
| A�� | Z���Ե��� | B�� | Z���Ա�ʾΪ��XY2 | C�� | X�γ�+2�������� | D�� | Z�ĵ���ʽΪ |
| A�� | Fe | B�� | Mg | C�� | P | D�� | Na |
| A�� | ��������ȼ�ϵ�ص�������Ӧ��H2-2e-+2OH-�T2H2O | |
| B�� | �õ���ʽ��ʾNaCl���γɹ��̣�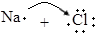 ��Na+[ ��Na+[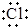 ]- ]- | |
| C�� | ������������ͨ��������Ƽ�����Һ�У�SO2+C1Oһ+2OH-�TSO42-+Cl-+H2O | |
| D�� | ����β��ϵͳ�Ĵ�ת�����ɽ���NO�ȵ��ŷţ�2CO+2NO$\frac{\underline{\;����\;}}{\;}$2CO2+N2 |
| A�� | �Ҵ������ᶼ�������Ʒ�Ӧ�������� | |
| B�� | ��֬�����ۡ���ά�ض�������Ȼ�߷��ӻ����� | |
| C�� | �ױ�����ϩ��������ˮ������ѧ��Ӧ�����ˮ��ɫ | |
| D�� | ��������Һ����CuSO4��Һ����������һ�������ڵ����ʵ����� |

 �ң�
�ң�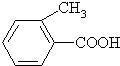 ����
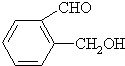
����
 ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺
ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺