��Ŀ����
1�����л�ѧ���ﲻ����ȷ���������ʵ���ǣ�������| A�� | ��������ȼ�ϵ�ص�������Ӧ��H2-2e-+2OH-�T2H2O | |
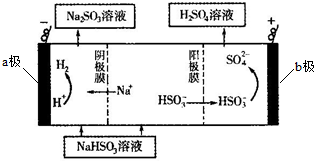
| B�� | �õ���ʽ��ʾNaCl���γɹ��̣�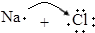 ��Na+[ ��Na+[ ]- ]- | |
| C�� | ������������ͨ��������Ƽ�����Һ�У�SO2+C1Oһ+2OH-�TSO42-+Cl-+H2O | |
| D�� | ����β��ϵͳ�Ĵ�ת�����ɽ���NO�ȵ��ŷţ�2CO+2NO$\frac{\underline{\;����\;}}{\;}$2CO2+N2 |
���� A����������ȼ�ϵ���У�������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
B��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl��
C����������ʹ������Ʒ���������ԭ��Ӧ���������ƺ�HCl��
D���ڴ��������£�CO��NO��Ӧ���ɶ�����̼�͵�����
��� �⣺A����������ȼ�ϵ���У�������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��������ӦʽΪH2-2e-+2OH-�T2H2O����A����
B��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl���������γɹ���Ϊ ��Na+[
��Na+[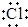 ]-����B��ȷ��
]-����B��ȷ��
C����������ʹ������Ʒ���������ԭ��Ӧ���������ƺ�HCl�����ӷ���ʽΪSO2+C1O-+2OH-�TSO42-+Cl-+H2O����C��ȷ��
D���ڴ��������£�CO��NO��Ӧ���ɶ�����̼�͵������Ӷ����ͻ�����Ⱦ����Ӧ����ʽΪ��2CO+2NO$\frac{\underline{\;����\;}}{\;}$2CO2+N2����D��ȷ��
��ѡA��
���� ���⿼�黷����Ⱦ��������ԭ��Ӧ������ʽ���缫��Ӧʽ����д��֪ʶ�㣬���ؿ���������ۣ���ȷ���ʵ������ǽⱾ��ؼ���ע��ԭ��ط�ӦʽҪ��ϵ������Һ�������д���״�ѡ����C��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ����������Һ�백ˮ��Ӧ��ȡ�������� | |
| B�� | ����ʯ��ˮʢװ���ò������������Լ�ƿ�� | |
| C�� | ������������ȼ�ղ�����ɫ���� | |
| D�� | ������ˮͨ����������ɫ�Լ�ƿ |
| A�� | NaHCO3��Һ�����CO32-+2 H+=H2O+CO2�� | |
| B�� | ��������Һ��ͭ��Cu+Ag+=Cu2++Ag | |
| C�� | ��������ˮ��Ӧ��K+H2O�TK++OH-+H2�� | |
| D�� | �ô����ˮ����2CH3COOH+CaCO3=Ca2++2CH3COO-+H2O+CO2�� |
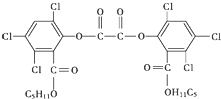 ��ħ�����������������ֳ����յ���Ⱦ����ħ��������ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ�㷢��ӫ�⣬���������CPPO���ṹ��ʽ��ͼ������˵������ȷ���ǣ�������
��ħ�����������������ֳ����յ���Ⱦ����ħ��������ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ�㷢��ӫ�⣬���������CPPO���ṹ��ʽ��ͼ������˵������ȷ���ǣ�������| A�� | CPPO������ˮ | |
| B�� | CPPO���ڷ�����Ҳ���ڸ߷��ӻ����� | |
| C�� | 1 mol CPPO��������ȫ��Ӧ����Ҫ10 mol H2 | |
| D�� | 1 mol CPPO��NaOHϡ��Һ��Ӧ�������DZ�������ԭ��ˮ�⣩���������4 mol NaOH |
| A�� | S��s��+O2��g����SO2��g��+Q1kJ��S��g��+O2��g����SO2��g��+Q2kJ | |
| B�� | H2��g��+$\frac{1}{2}$O2��g����H2O��l��+Q1kJ��H2��g��+$\frac{1}{2}$O2��g����H2O��g��+Q2kJ | |
| C�� | NaOH��aq��+HCl��aq����NaCl��aq��+H2O��l��+Q1kJ NaOH��aq��+HAc��aq����NaAc��aq��+H2O��l��+Q2kJ | |
| D�� | H2��g��+Cl2��g����2HCl��g��+Q1kJ��H2��g��+I2��g����2HI��g��+Q2kJ |
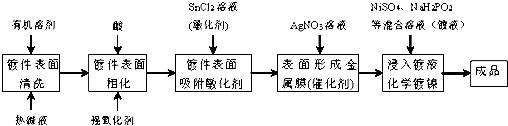
�ش��������⣺
��1����ѧ�����ƱȽϣ��ŵ�֮һ�Dz���ͨ�磮
��2���Ƽ��������ȼ�Һ��ϴ�������dz�ȥ�Ƽ��������ۣ��Ƽ�����ֻ���Ŀ������ǿ��ˮ�Լ�����Ӵ������
��3���Ƽ�����AgNO3��Һ�Ƽ�����������SnCl2��AgNO3��ԭ�����д����ԵĽ���������Ӧ�Ļ�ѧ����ʽ��2SnCl2+4AgNO3�T4Ag+SnCl4+Sn��NO3��4��
��4������ʱ����Һ�е�Ni2+��H2PO2-�ڴ������Ϸ�Ӧ��������ͬʱ������ǿ��H3PO3������������ʵ�����ȵ��������÷�Ӧ�����ӷ���ʽ��2H2O+Ni2++2H2PO2-=Ni++H2��+2H3PO3��
��5����ѧ��ij�ֽ���ʱ����Ӧʱ����Ʋ��ȵ�ʵ�����ݼ�¼�����ʾ��
| ��Ӧʱ��t/s | 1 | 4 | 9 | 16 |
| �Ʋ���y/nm | a | 2a | 3a | 4a |
��6����ѧ������Һ�к���Ni 2+����Ⱦ���ת��Ϊ������ȥ����֪25�棬Ksp[Ni��OH��2]=2.0��10-15����������ʹ��Һ��pH=10��������ķ�Һ����Ԫ�صĺ���Ϊ1.2��10-2mg•L-1��
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ksp��Ka | Ksp=1.8��10-10 | Ksp=9.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ����Һ�У���������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ����������Һ��ͨ������CO2�����ӷ���ʽΪ��2ClO-+CO2+H2O=CO32-+2HClO | |
| C�� | ��0.1 mol•L-1CH3COOH��Һ�еμ�NaOH��Һ����c��CH3COOH����c��CH3COO-��=5��9����ʱ��Һ��pH=5 | |
| D�� | ��Ũ�Ⱦ�Ϊ1.0��10-3 mol•L-1��KCl��K2CrO4�����Һ�еμ�1.0��10-3 mol•L-1��AgNO3��Һ��CrO42-���γɳ��� |