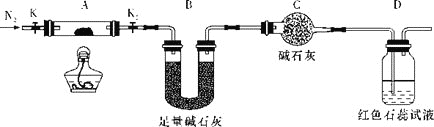
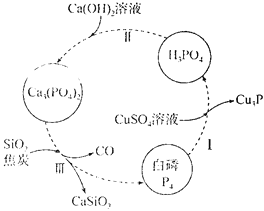
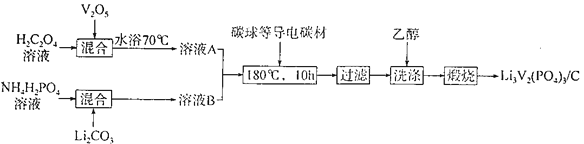
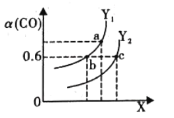
��Ŀ����
����Ŀ���������ʵ���A��B�����2L���ܱ������з�����Ӧ��3A(g)+B(g)xC(g)+D(g)����4min���D��Ũ��Ϊ0.4 mo1��L-1��C��ƽ����Ӧ����Ϊ0.lmo1��L-1��min-1��c(A)��c(B)=3��5������˵������ȷ����
A.x��ֵ��1B.4minĩ��A��ת����Ϊ60%
C.��ʼʱA��Ũ��Ϊ2.4mol��L-1D.4min��v(B)=0.1 mol��L-1��min-1
���𰸡�B
��������

(a-1.2): (a-0.4) = 3:5
���a = 2.4
A. ![]() ��x =1����A��ȷ�����������⣻
��x =1����A��ȷ�����������⣻
B. 4minĩ�� A��ת����Ϊ![]() ����B���������⣻
����B���������⣻
C. ���ݷ����ó���ʼʱA��Ũ��Ϊ2.4mol��L-1����C��ȷ�����������⣻
D. ����B��Cϵ�����������ȣ��ó�4min��v(B)=0.1 mol��L-1��min-1����D��ȷ�����������⣻
��������������ΪB��

��ϰ��ϵ�д�
�����Ŀ