��Ŀ����
4��ԭ���γɻ�����ʱ�������Ƽ������ö����ʵĽṹ�����ʻ����Ӱ�죮�Իش���1��BF3���ӵĿռ乹��Ϊƽ�������Σ�[BF4]-�Ŀռ乹��Ϊ���������Σ�
��2��̼ԭ����4���۵��ӣ����л��������м۵��Ӿ�����ɽ������ӻ���ʽ��һ����ͬ���ױ���
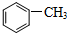 ����̼ԭ�ӵ��ӻ���ʽ��SP2��SP3���ױ��ܱ�����KMnO4��Һ�����ɱ����ᣨ
����̼ԭ�ӵ��ӻ���ʽ��SP2��SP3���ױ��ܱ�����KMnO4��Һ�����ɱ����ᣨ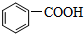 ����������ķе����Ը��ڼױ�������Ҫԭ���DZ�������Ӽ���������
����������ķе����Ը��ڼױ�������Ҫԭ���DZ�������Ӽ�����������3��H+ͨ����λ����H2O����γ�H3O+��д��H3O+�ĵ���ʽ
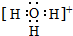 ��H3O+�ļ��Ǵ��ڣ�����ڡ�����С�ڡ����ڡ���ˮ���ӵļ��ǣ�
��H3O+�ļ��Ǵ��ڣ�����ڡ�����С�ڡ����ڡ���ˮ���ӵļ��ǣ���4���ȵ���ԭ����ָԭ��������ͬ���۵���������ͬ�����Ӿ������ƵĻ�ѧ����������֪��CO2��N2O��Ϊ�ȵ����壬��N2O��Oԭ��ֻ��1��Nԭ�ӣ���д��N2O�ĽṹʽN=N=O��
���� ��1�����ݼ۲���Ӷ���=�ɼ����Ӷ���+�µ��Ӷ����жϣ�
��2����������һ����м�������Cԭ���γ�4�����ۼ������Ӽ�������ʱ�е�ϸߣ�
��3��H3O+Ϊ�����ӣ�Oԭ����Χ8�����ӣ��µ��ӶԺͳɼ����Ӽ���ų���С�ڹµ��Ӷ�֮����ų�����
��4���ȵ�����Ľṹ���ƣ����ݶ�����̼�Ľṹʽ������
��� �⣺��1��BF3������Bԭ�ӵļ۲���Ӷ���=3+$\frac{1}{2}$��3-3��1��=3������sp2�ӻ������ӵĿռ乹��Ϊƽ�������Σ�[BF4]-��Bԭ�ӵļ۲���Ӷ���=4+$\frac{1}{2}$��3+1-4��1��=4������sp3�ӻ����ռ乹��Ϊ�������壬
�ʴ�Ϊ��ƽ�����ǣ��������壻
��2����������һ����м���Cԭ�ӵļ۲���Ӷ���Ϊ3������sP2�ӻ�������Cԭ���γ�4�����ۼ����۲���Ӷ���Ϊ4������sp3�ӻ�����������Ӽ�������ʱ�е�ϸߣ����Ա�����ķе����Ը��ڼױ���
�ʴ�Ϊ��sP2��sP3����������Ӽ���������
��3��H3O+Ϊ�����ӣ�Oԭ����Χ8�����ӣ������ʽΪ��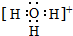 ���µ��ӶԺͳɼ����Ӽ���ų���С�ڹµ��Ӷ�֮����ų�����H3O+�к���һ���µ��Ӷԣ�H2O�к������Թµ��Ӷԣ�����H3O+��H-O-H���Ǵ���H2O��H-O-H���ǣ�
���µ��ӶԺͳɼ����Ӽ���ų���С�ڹµ��Ӷ�֮����ų�����H3O+�к���һ���µ��Ӷԣ�H2O�к������Թµ��Ӷԣ�����H3O+��H-O-H���Ǵ���H2O��H-O-H���ǣ�
�ʴ�Ϊ��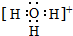 �����ڣ�
�����ڣ�
��4���ȵ�����Ľṹ���ƣ�CO2��N2O��Ϊ�ȵ����壬������̼�ĽṹʽΪO=C=O����N2O�ĽṹʽΪ��N=N=O��
�ʴ�Ϊ��N=N=O��
���� ���⿼�������ʽṹ�����ʣ��漰ԭ���ӻ���ʽ���жϡ����ӿռ����͵��жϡ�����ʽ���ȵ������֪ʶ�㣬���ݼ۲���ӶԻ�������ȷ��ԭ���ӻ���ʽ�����ӿռ����͵��жϣ�

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�| �� | �� | �� | �� | |
| ��һ�����ܣ�kJ/mol�� | 1681 | 1251 | 1140 | 1008 |
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
| A�� | ���ԣ�Al��OH��3��Mg��OH��2��NaOH | B�� | ��ԭ�ԣ�HCl��HBr��HI | ||
| C�� | ���ԣ�HClO4��H2SO4��H3PO4 | D�� | �ȶ��ԣ�HI��HBr��HCl |
| A�� | C2H4 | B�� | CaCl2 | C�� | Na2O2 | D�� | NH4NO3 |
 �����ס���Ϊͬ����Ԫ�أ������⻯��RH3��NH3��PH3��AsH3����ij��������R�ĺ˵�����ı仯������ͼ��ʾ����Y��ɱ�ʾ���⻯�RH3�������ǣ�������
�����ס���Ϊͬ����Ԫ�أ������⻯��RH3��NH3��PH3��AsH3����ij��������R�ĺ˵�����ı仯������ͼ��ʾ����Y��ɱ�ʾ���⻯�RH3�������ǣ�������| A�� | �ȶ��� | B�� | �е� | C�� | R-H���� | D�� | ���Ӽ������� |

| A�� | ԭ��������15 | B�� | ���Ƿǽ���Ԫ�� | ||
| C�� | ��ԭ���������5������ | D�� | ��Ԫ���ڵؿ��еĺ���Ϊ30.97% |
| A�� | ���������������������Һ��Ӧ��SO2+2OH-=SO32-+H2O | |
| B�� | ����ϡ������봿���У�CO32-+2H+=H2O+CO2�� | |
| C�� | ͭ����ϡ������Һ��Ӧ��Cu+2H+=Cu2++H2�� | |
| D�� | ���Ȼ�����Һ�еμӹ����İ�ˮ��Al3++3NH3•H2O=Al��OH��3��+3NH4+ |


 ��R1��R2��R3Ϊ��������ԭ�ӣ�
��R1��R2��R3Ϊ��������ԭ�ӣ� �����ⶨ�����صķ�������2������������ȩ���ɽ����صĻ�ѧ����ʽΪ
�����ⶨ�����صķ�������2������������ȩ���ɽ����صĻ�ѧ����ʽΪ ��
��