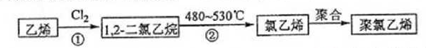
题目内容
9.碘是人体必须的元素之一,海洋植物如海带、海藻中含有丰富的、以碘离子形式存在的碘元素.在实验室中,从海藻里提取碘的流程和实验装置如下: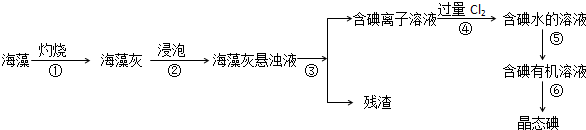
(1)指出上述提取碘的过程中有关实验操作的名称:
步骤③过滤,步骤⑤萃取
(2)写出步骤④对应反应的离子方程式:Cl2+2I-═I2+2Cl-
(3)步骤④除了加入过量Cl2,下列氧化剂最好选用B(填代号).
A.浓硫酸 B.H2O2溶液 C.KMnO4溶液
理由是过氧化氢是绿色氧化剂,在氧化过程中不会引进杂质、不产生污染
(4)提取碘的过程中,可供选择的有机试剂是CD.(填编号)
A.酒精 B.醋酸 C.四氯化碳 D.苯
(5)为了使海藻灰中的碘离子转化为碘的有机溶液,即完成步骤③至⑤,实验室里有烧杯、玻璃棒、集气瓶、酒精灯、导管、圆底烧瓶、石棉网、以及必要的夹持仪器和物品,尚缺少的玻璃仪器是分液漏斗、普通漏斗.
分析 (1)分离固体和液体用过滤;用一种溶剂把溶质从它跟另一种溶剂所组成的溶液里提取出来用萃取;
(2)氯气具有氧化性,能将碘离子氧化为碘单质;
(3)氢离子和过氧化氢将碘离子氧化为单质碘,在氧化过程中不会引进杂质、不产生污染;
(4)萃取剂的选取标准:和原溶剂不互溶,溶质在萃取剂中的溶解度大于在原溶剂中的溶解度,萃取剂和溶质不反应;
(5)分液漏斗和普通漏斗的作用分别为萃取和过滤.
解答 解:(1)分离难溶性固体和溶液采用过滤的方法,海藻灰难溶于水,碘离子易属于水,所以步骤③为过滤,碘在有机溶剂中的溶解度大于在水溶液中的溶解度,所以步骤⑤可以采用萃取的方法萃取出碘水中的碘,
故答案为:过滤;萃取;
(2)氯气具有氧化性,能将碘离子氧化为碘单质,步骤④氯气氧化碘离子,即:Cl2+2I-=2Cl-+I2,
故答案为:Cl2+2I-=2Cl-+I2;
(3)加入氢离子和过氧化氢起的作用为氧化剂,将碘离子转化为单质碘,离子方程式为2H++2I-+H2O2═I2+2H2O,过氧化氢是绿色氧化剂,在氧化过程中不会引进杂质、不产生污染,
故答案为:B;过氧化氢是绿色氧化剂,在氧化过程中不会引进杂质、不产生污染;
(4)酒精、醋酸都与水溶液互溶,溶液不分层,无法用于分液操作分离碘单质,碘易溶于四氯化碳和苯,且四氯化碳和苯都符合萃取剂选取标准,所以可以用四氯化碳或苯作萃取剂,
故答案为:CD;
(5)③缺少用于过滤的普通漏斗,在分液操作中,主要属于的仪器为分液漏斗,所以⑤缺少用于萃取使用的分液漏斗,
故答案为:分液漏斗、普通漏斗.
点评 本题考查海水资源综合利用,侧重考查混合物的分离和提纯、实验基本操作能力的考查,明确物质的性质及实验操作规范性是解本题关键,知道常见混合物分离和提纯方法及选取方法,题目难度不大.

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案| A. | 若容器容积不变,升高温度,各气体的相对分子质量一定增大 | |
| B. | 若从正反应开始,平衡时A、B的转化率相等,则A、B的物质的量之比为a:b | |
| C. | 达到平衡时,有amol A消耗的同时有b mol B生成 | |
| D. | 若容器为体积不变的密闭容器且a+b=c+d,则当升高容器内温度时平衡向左移动,容器中气体的压强增大 |
①HCl比H2S稳定 ②HClO4酸性比H2SO4强 ③Cl2能与H2S反应生成S.
| A. | ②③ | B. | ①② | C. | ①③ | D. | ①②③ |
| A. | Zn负极,并被还原 | B. | Ag2O正极,并被还原 | ||
| C. | 电解质溶液为碱性 | D. | Zn正极,并被氧化 |
 室温下,向20.00mL 1.000mol•L-1氨水中滴入1.000mol•L-1盐酸,溶液pH和温度随加入盐酸体积变化曲线如图所示.下列有关说法错误的是( )
室温下,向20.00mL 1.000mol•L-1氨水中滴入1.000mol•L-1盐酸,溶液pH和温度随加入盐酸体积变化曲线如图所示.下列有关说法错误的是( )| A. | a、d两点的溶液,水的离子积Kw(a)>Kw(d) | |
| B. | 将此氨水稀释,溶液的导电能力减弱 | |
| C. | c点时消耗盐酸体积V(HCl)<20 mL | |
| D. | 室温下,a点的氨水电离常数为102(a−14)1−10a−14102(a−14)1−10a−14 mol•L-1 |
| A. | 分子中不一定含有氧元素 | B. | 分子中一定含有氧元素 | ||
| C. | 在反应中易失电子的物质 | D. | 是反应生成的物质 |
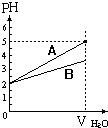 pH值等于2的两种酸溶液A和B,分别加水稀释1000倍,其pH值与所加水的体积变化趋势示意图如图所示,则下列结论正确的是:( )
pH值等于2的两种酸溶液A和B,分别加水稀释1000倍,其pH值与所加水的体积变化趋势示意图如图所示,则下列结论正确的是:( )| A. | 等体积pH=2的两种酸中和NaOH的能力酸A比酸B强 | |
| B. | A为弱酸,B为强酸 | |
| C. | 酸B的摩尔浓度比酸A大 | |
| D. | A比B易电离 |