��Ŀ����
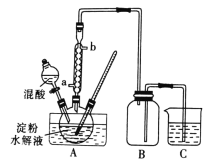
����Ŀ����(Se)��ͭ(Cu)�������������й㷺��Ӧ�á������������������ϡ��������ҵ�Ĵ�����Ҳ�Ƕ���������Ӫ��Ԫ�غͶ�ֲ�������Ӫ��Ԫ�صȡ��Ȼ���ͭ(CuCl)�㷺Ӧ���ڻ�����ӡȾ����Ƶ���ҵ��CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ���ڳ�ʪ��������ˮ���������Ժ���ͭ(��Ҫ�ɷ���Cu������CuO)Ϊԭ�ϣ���������������ֽ⼼������CuCl�Ĺ��չ���������ʾ��
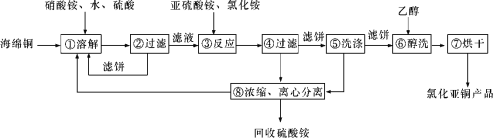
��ش��������⣺
(1)��������еõ�����������ֻ��һ�֣������Ļ�ѧʽ��____________��
(2)д�����������Ҫ��Ӧ�����ӷ���ʽ��____________________________________��
(3)����ݰ�����pH��2����Һ��ϴ��ˮϴ������������ϴ���õ�����__________(д�������)��
(4)���������У�����͢ߵ�������_____________��
(5)SeΪ��A��Ԫ�أ����Ҷ���������ͭ������ˮ��Һ������������(Na2SeSO3)��Һ��Ӧ�ɻ����������ͭ�����������ƻ�������Se�ľ��ƣ�д������������(Na2SeSO3)��H2SO4��Һ��Ӧ�õ������Ļ�ѧ����ʽ��_____��
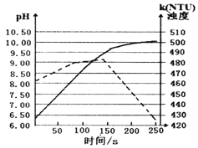
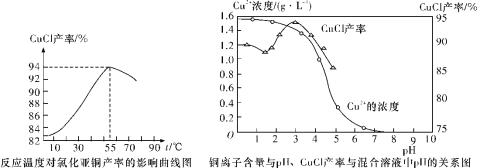
(6)�Ȼ���ͭ�������¶ȡ���ҺpH��ϵ����ͼ��ʾ����ͼ���������̻������Ȼ���ͭ�Ĺ����У��¶ȹ���Ӱ��CuCl���ʵ�ԭ����____________________________________���¶ȹ��ߡ�pH����Ҳ��Ӱ��CuCl���ʵ�ԭ����_______________________________��
(7)��NaHS����ˮ�����ij����������Դ�����ҵ��ˮ�е�Cu2������֪��25��ʱ��H2S�ĵ���ƽ�ⳣ��Ka1��1.0��10��7��Ka2��7.0��10��15��CuS���ܶȻ�ΪKsp(CuS)��6.3��10��36����ӦCu2��(aq)��HS��(aq) ![]() CuS(s)��H��(aq)��ƽ�ⳣ��K��__________(�������1λС��)��
CuS(s)��H��(aq)��ƽ�ⳣ��K��__________(�������1λС��)��
���𰸡�CuSO4 2Cu2+ +SO32- +2Cl- + H2O=2CuCl��+SO42- +2H+ ���� ʹCuCl�����ֹ��ˮ������ Na2SeSO3��H2SO4=Na2SO4��Se����SO2����H2O �¶ȹ��ͷ�Ӧ������ �¶ȹ��ߡ�pH����������CuO��Cu2Oת�������¶ȹ��ߣ����(�Ȼ�泥��������)�����ȷֽ�(�δ�һ�㼴��) 1.1��1021
��������
������������������Ӿ��������ԣ�����������ͭ(��Ҫ�ɷ���Cu������CuO)��������ͭ�����˺�����Һ�м���������立���������ԭ��Ӧ����CuCl������2Cu2+ +SO32- +2Cl- + H2O=2CuCl��+SO42- +2H+���õ���CuCl��������ϴ��ˮϴ�������Ҵ�ϴ�ӣ���ɵõ��Ȼ���ͭ��
(1) ����������������������Ӿ��������ԣ�������Cu����CuSO4���ʴ�Ϊ: CuSO4��
(2)ͭ������������立���������ԭ��Ӧ����CuCl�����������Ҫ��Ӧ�����ӷ���ʽΪ2Cu2+ +SO32- +2Cl- + H2O=2CuCl��+SO42- +2H+��
(3) CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ���ڳ�ʪ��������ˮ����������ֹCuCl�ܽ��������������ʣ�����Ӧ�������ᣬ���ܼ���������������ᣬҲ���ܼ������ᣬ�ʴ�Ϊ:���
(4) �����Ϊ��ϴ�������Ϊ��ɣ����Ҵ��е�ͣ��ӷ������Ҵ�ϴ�ӣ��ɿ��ٳ�ȥ��������ˮ�֣���ֹCuClˮ�⡢�������ʴ�Ϊ:��ϴ�����ڼӿ�ȥ��CuCl����ˮ�ַ�ֹ��ˮ��������
��5������������(Na2SeSO3)��H2SO4��Һ��Ӧ�õ�������ͬʱ���������ơ����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Na2SeSO3��H2SO4=Na2SO4��Se����SO2����H2O��
��6����ͼ���������̻������Ȼ���ͭ�Ĺ����У��¶ȹ���Ӱ��CuCl���ʵ�ԭ�����¶ȹ��ͷ�Ӧ���������¶ȹ��ߡ�pH����Ҳ��Ӱ��CuCl���ʵ�ԭ�����¶ȹ��ߡ�pH����������CuO��Cu2Oת�������¶ȹ��ߣ����(�Ȼ�泥��������)�����ȷֽ⣻
��7����ӦCu2��(aq)��HS��(aq) ![]() CuS(s)��H��(aq)��ƽ�ⳣ��K=
CuS(s)��H��(aq)��ƽ�ⳣ��K=![]() ��
��

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
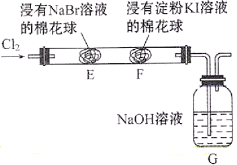
С����ȫ�ܼ��ϵ�д�����Ŀ��ʵ��С��Ϊ��֤NO2��ˮ��Ӧ�IJ������ͼ��ʾװ�ý���ʵ�飨�г�װ������ȥ���������Ѽ��飩��
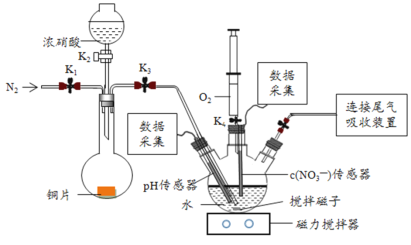
��ʵ����̣�
ʵ�鲽�� | ʵ������ |
��.��K1��K3��K5���ر�K2��K4��ͨ��һ��ʱ��N2���ر�K1 | ���� |
��.��K2����������ŨHNO3��ͬʱ��pH��������NO3-����������¼���� | Բ����ƿ�з�Ӧ���ң�ͭƬ���ܽ⣬��Һ��Ϊ����ɫ�� ��Ƭ�̺�����ƿ�ڵĵ��ܿ�������ð�� |
III.5min��K4����ע��������������ע������ƿ���ر�K4 | ����ƿ�ڵ��������ɫ��Ϊdz����ɫ |
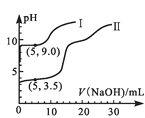
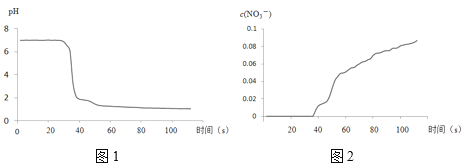
����II�У���������¼������ͼ��ʾ��
�����ͼ����ۣ�
��1��NO2��ˮ��Ӧ�����ӷ���ʽΪ___��
��2������I�У�ͨ��N2��Ŀ����___��
��3��������II��Բ����ƿ�ڵ�ʵ������������___��
��4����ʵ����֤NO2��ˮ��Ӧ�����ʵ��֤�ݰ���___������ţ���
A.Բ����ƿ����Һ��Ϊ����ɫ
B.����ƿ�ڵ��������ɫ��Ϊdz����ɫ
C.pH��������¼��������
D.NO3-��������¼��������
��5����ͬѧ��Ϊ��ʵ�鲻�Ͻ�����ΪҲ���ܵ��´���������¼�����ݽ��___��