��Ŀ����
����˵����ȷ����
| A�������£�ij��Һ����ˮ�������c(H+)��1��10-amo1��L�C1����a��7ʱ�������Һ����ΪNaHSO4��Һ |
| B�������£��к�ͬ�����ͬpH�����ᡢ����ʹ���������ͬŨ�ȵ�NaOH��Һ�������ϵ��V�����ᣩ��V�����ᣩ��V�����ᣩ |
| C��25��ʱ����֪Ka(CH3COOH)=1.7��10-5mo1��L�C1��Ka(C6H5OH) =1.0��10-10mo1��L�C1�� Ka1(H2CO3) = 4.2��10-7mo1��L�C1 ��Ka2(H2CO3) ��5.6��10-11mo1��L�C1pH��ȵĢ�CH3COONa ��C6H5ONa ��NaHCO3��Һ�У�c(Na+)��С��ϵ���ڣ��ۣ��� |
| D�������£���Na2CO3��Һ�м�������BaSO4��ĩ�����ˣ���ϴ���ij����м���ϡ���������ݲ�����˵��������Ksp��BaSO4��>Ksp��BaCO3�� |
C
�����������;Aѡ�����ˮ�����ӻ�����Ϊ1��10-14mo1��L�C1����a��7�����Һ��������Ũ�ȴ�������������Ũ�ȣ����Ը���Һ�����ԣ�����Aѡ������ȷ�ġ�Bѡ�һ���������������ȫ��������������ӣ���ǿ�������Һ���������������ᣬ������ȫ���롣�����ǵ�pH��ȣ�������Ĵ�����������ģ����ŵ����ᣬ��Ҫ������������ٵġ�����Bѡ���Ǵ���ġ�Dѡ��ܽ�ȳ���Խ��˵��������Խ�����ܽ���ˮ�С�Dѡ��Ӧ����Ksp��BaSO4���Ƚ�С��
���㣺����������Һ�����֪ʶ�㡣

��ϰ��ϵ�д�
�����Ŀ
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ__________________________________________��
��2��ʵ��ٵ�Ŀ����______________________________________________________��ʵ���еμ�FeCl3��Һ��Ŀ����_________________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������__________________________________����ʵ�������ṩ�ļ����Լ�����
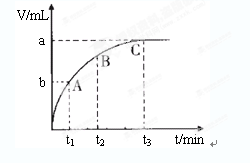
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ������������______________________��
��֪25 ��ʱ��AgCl ���ܶȻ�Ksp=1.8��10��10��������˵����ȷ���ǣ� ��
| A����AgClˮ��Һ�м������ᣬKspֵ��� |
| B��AgNO3��Һ��NaCl��Һ��Ϻ����Һ�У�һ����c(Ag��)=c(Cl��) |
| C���¶�һ��ʱ������Һ��c(Ag��)��c(Cl��)=Kspʱ������Һ�б���AgCl�ij������� |
| D����AgCl���뵽KI��Һ�У�AgClת��ΪAgI����ΪAgCl�ܽ�ȴ���AgI |
25��ʱ��ˮ�д��ڵ���ƽ�⣺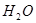

 ������������ȷ����
������������ȷ����
A����ˮ���ȣ�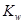 ���� ���� ���� ���� |
B����ˮ�м�������NaHSO4���壬c(H+)���� ���� ���� |
C����ˮ�м�������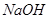 ���壬ƽ�������ƶ��� ���壬ƽ�������ƶ���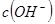 ���� ���� |
D����ˮ�м�������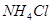 ���壬ƽ�������ƶ��� ���壬ƽ�������ƶ���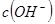 ���� ���� |
����˵����ȷ���� �� ��
| A��ǿ�������Һ�ĵ�������һ�������������Һ�ĵ�������ǿ |
| B����ΪCH3COOH��������ʣ�HCl��ǿ����ʣ������к͵���������ʵ���Ũ�ȵĴ��������ʱ���кʹ������ĵ�NaOH���������ĵ�NaOH������ |
| C�����ʵ���Ũ����ͬ����������Һ��������Һ��PO43-�����ʵ���Ũ����ͬ |
| D������Zn�ֱ�͵�����������ʵ���Ũ�ȵ�����ʹ��ᷴӦʱ������H2������ͬ���ų�H2�����ʲ��� |
�����£�0.1mol��L��1CH3COONa��Һ�У���Ũ�ȼ��ϵ��ȷ����
| A��c(Na+)=c(CH3COO��) ��c(OH��)=c(H+) |
| B��c(OH��)=c(H+)+ c(CH3COOH) |
| C��c(Na+) + c(H+)= c(CH3COO��) +c(OH��) |
| D��c(CH3COOH) + c(CH3COO��) = c(Na+)+ c(H+) |
��֪KHSO3��Һ�������ԡ���0.1mol��L-1KHSO3��Һ�У����й�ϵ��ȷ����
| A��c(K+)+ c(H+) ��c(HSO3-)+ c(OH-)+ c(SO32-) |
| B��c(HSO3-) + c(SO32-) = 0.1mol��L-1 |
| C��c(SO32-) �� c(H2SO3) |
| D��c(K+) = c(H2SO3) + c(HSO3-) + c(SO32-) |
����˵����ȷ����
| A��ˮ�����ӻ�����KWֻ���¶��йأ�������ᡢ���һ����Ӱ��ˮ�ĵ���̶� |
| B��Ksp���������ܵ���ʵ����ʺ��¶��йأ�������Һ��������ӵ�Ũ���й� |
| C�������£���10mL PH=3�Ĵ�����Һ�м�ˮϡ�ͺ���Һ��C(CH3COO--)/C(CH3COOH) C(OH--)��ֵ��С |
| D�������£���0.10 mol��L��1��NH3��H2O��Һ�м�������NH4Cl���壬��ʹ��Һ��pH��С��c(NH)/c(NH3��H2O)��ֵ���� |
������,����0.1 mol.L-1pH= 10��Na2CO3��Һ������˵����ȷ����
| A��ͨ��CO2����ҺpH��С |
B������NaOH���壬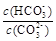 ���� ���� |
| C����ˮ�����c(OH-) = 1��10-10 mol.L-1 |
| D����Һ�У�c(CO32-)>c(OH-)>c(HCO3-) |