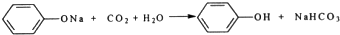��Ŀ����
4��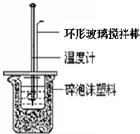 ���к��ȵIJⶨ��ʵ�鲽��Ϊ��
���к��ȵIJⶨ��ʵ�鲽��Ϊ��������Ӧװ�ã���ͼ����
����һ����Ͳ��ȡ40mL 0.50mol/L���ᣬ����С�ձ��У������¶ȼƲ���������¶ȣ���¼��Ȼ����¶ȼ��ϵ�����ˮ��ϴ�ɾ���
������һ����Ͳ��ȡ40mL 0.55mol/LNaOH��Һ�������¶ȼƲ���NaOH��Һ���¶ȣ���¼��
�ܰ��¶ȼƺ�___����С�ձ��������У�������Ͳ�е�NaOH��Һһ�ε���С�ձ���ע�ⲻҪ�������棩���û��β�����������������Һ����ȷ��ȡ�����Һ������¶ȣ�
��___��
����ʵ�����ݼ����к��ȣ�
��ش���������
��1����ȫ�ڢܲ�����ȱ�������β����������
��2����ȫ�ڢݲ����������ظ�ʵ�鲽���-�ܲ�2-3�Σ���¼�����
��3���ڢڲ���������¶ȼ��ϵ�����ˮ��ϴ�ɾ�ֱ�Ӳ���NaOH��Һ���¶ȣ����õġ�Hƫ���ƫ��ƫС������Ӱ�족����
��4��������Ϊ0.50mol/L����0.55mol/LNaOH��Һ���ܶȶ���1g/mL���кͺ����ɵ���Һ�ı�����c=4.18J/��g•�棩����ʼƽ���¶�Ϊt1�棬��Ӧ������¶�Ϊt2�棬���к��ȣ����û�����H=-$\frac{80��4.18��1{0}^{-3}����{t}_{2}-{t}_{1}��}{0.02}$kJ/mol��
��5��0.50L 1.00mol/L H2SO4��Һ��1.00L 1.00mol/L NaOH��Һ��ȫ��Ӧ���ų�57.16kJ��������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.16KJ/mol��
��6�����ܣ���ܡ����ܡ�����ϡBa��OH��2��ϡ������������NaOH��Һ�������ǻ��ᷢ��Ba2+��SO42-�ķ�Ӧ���÷�ӦҲ������ЧӦ��
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2��Ϊ�˼���ʵ�����ʵ��Ӧ�ظ�2-3�Σ�
��3������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С��
��4������Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol������к��ȣ�
��5���������ʵ����������������Լ��Ȼ�ѧ����ʽ����д���������
��6���������������ᷴӦ���������ᱵ���������ɳ����Ĺ����л��������仯��Ӱ��ⶨ�����
��� �⣺��1���ɰ��¶ȼƺͻ��β������������С�ձ��������У�������Ͳ�е�NaOH��Һһ�ε���С�ձ���ע�ⲻҪ�������棩���û��β�����������������Һ����ȷ��ȡ�����Һ������¶ȣ�
�ʴ�Ϊ�����β����������
��2��Ϊ�˼���ʵ�����ʵ��Ӧ�ظ�ʵ�鲽���-�ܲ�2-3�Σ���¼�����
�ʴ�Ϊ���ظ�ʵ�鲽���-�ܲ�2-3�Σ���¼�����
��3������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС�����к���ƫ��
�ʴ�Ϊ��ƫ��
��4����ʼƽ���¶�Ϊt1�棬��Ӧ������¶�Ϊt2�棬�¶Ȳ�Ϊ����t2-t1���棬40mL 0.50mol/L������40mL 0.55mol/LNaOH��Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.04L��0.50mol/L=0.02mol����Һ������Ϊ80g���¶ȱ仯��ֵΪ����t2-t1���棬������0.02molˮ�ų�������ΪQ=m•c•��T=80g��4.18��10-3kJ/��g•�棩����t2-t1���棬����ʵ���õ��к��ȡ�H=-$\frac{80��4.18��1{0}^{-3}����{t}_{2}-{t}_{1}��}{0.02}$kJ/mol��
�ʴ�Ϊ��-$\frac{80��4.18��1{0}^{-3}����{t}_{2}-{t}_{1}��}{0.02}$kJ/mol��
��5��0.50L 1.00mol/L H2SO4��Һ��1.00L 1.00mol/L NaOH��Һ��ȫ��Ӧ���ų�57.16kJ��������������1molˮʱ�ų�57.3kJ�����������Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.16KJ/mol��
�ʴ�Ϊ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.16KJ/mol��
��6��������Ba��OH��2��Һ��Ӧ��������ˮ�⣬��������BaSO4�������÷�Ӧ�е������Ȼ�Ӱ�췴Ӧ�ķ�Ӧ�ȣ����Բ�����Ba��OH��2��Һ���������NaOH��Һ��������к��ȣ�
�ʴ�Ϊ�����ܣ����ᷢ��Ba2+��SO42-�ķ�Ӧ���÷�ӦҲ������ЧӦ��
���� ���⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��Ȳⶨԭ���Լ��ⶨ�к��ȵ��������⣬��������������ѧ�����Ӧ����ѧ֪ʶ��������

| A�� | C2H6 | B�� | C2H2 | C�� | C6H6 | D�� | C2H4O |
| ѹǿ��kPa�� | 200 | 500 | 1000 |
| B��Ũ�ȣ�mol/L�� | 0.04 | 0.1 | 0.27 |
��1��ѹǿ��200kPa���ӵ�500kPaʱ��ƽ�ⲻ�ƶ������������������������Ũ������ı�����ѹǿ����ı�����ͬ��
��2��ѹǿ��500kPa���ӵ�1000kPaʱ��ƽ�������ƶ��������������������ԭ�����Ϊ��ѹ��1000kPaʱ��C���ʱ���˷���̬���ʣ�
��3����ѧ����ʽ�еĻ�ѧ�������Ĺ�ϵ�ǣ�a=b+c���?����?����?����
| A�� | ��Ϊ���Ƕ��ǣ����Զ�����ζ | |
| B�� | ��Ϊ������ͬ�ķ���ʽ��C6H10H5��n�����Ի�Ϊͬ���칹�� | |
| C�� | ���߶���ˮ��Ϊ������ | |
| D�� | ���߶��������Ӫ��ʳ�� |
| A�� | Ħ����һ����λ�����ڼ����������������ӵ����� | |
| B�� | Ħ����һ�������� | |
| C�� | 1mol�κ��������������������Ŀ����� | |
| D�� | Ħ������������������������������������ |
| A�� | ��Һ��������Ũ�ȵĴ�С˳��c��S2-����c��OH-����c��HS-�� | |
| B�� | c��Na+��=2c��S2-��+c��OH-��+c��HS-�� | |
| C�� | c��Na+��=c��S2-��+c��HS-�� | |
| D�� | ����Һ�м�������NaOH���壬�ܴٽ�ˮ�ĵ��� |
| A�� | 56g N2 | B�� | 1.5molO2 | ||
| C�� | ��״����22.4LCl2 | D�� | 3.01��1023��SO2���� |
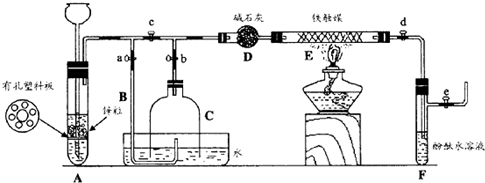
 2NH3��
2NH3��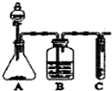 ��ʵ���ҳ�����ͼ�е�װ���Ʊ���Ȳ��������Ȳ������
��ʵ���ҳ�����ͼ�е�װ���Ʊ���Ȳ��������Ȳ������