��Ŀ����
9��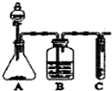 ��ʵ���ҳ�����ͼ�е�װ���Ʊ���Ȳ��������Ȳ������
��ʵ���ҳ�����ͼ�е�װ���Ʊ���Ȳ��������Ȳ��������1��ʵ�����Ʊ���Ȳ�Ļ�ѧ����ʽ��CaC2+2H2O��C2H2��+Ca��OH��2
��2��ʵ������У�A�з�Һ©��ͨ��ʹ�ñ���ʳ��ˮ����Ŀ���Ǽ�����ʯ��ˮ��Ӧ������
��3��B���Լ�������ͭ��Һ
��4����C���Լ�����ˮ�����Թ۲쵽����������Һ��ɫ
����˦����װ�û�������֤���ᡢ���ӡ�̼�������ǿ��
��1��A�й����Լ�������d��ѡ����ĸ����a��������b��̼��c��������d��С�մ�
��2��C�з�Ӧ�Ļ�ѧ����ʽ��
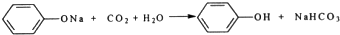
��3��Bװ���е��Լ�Ϊ����NaHCO3�����з�����Ӧ�����ӷ���ʽΪCH3COOH+HCO3-=CH3COO-+H2O+CO2������ͬѧ��Ϊû��Bװ�ã�Ҳ������֤���Ե�ǿ��������Ϊ�����𣿲������������������������
���� ��1����ʯ��ˮ��Ӧ������Ȳ���������ƣ�
��2����ʯ��ˮ��Ӧ�dz����ҡ���Ӧ���ʿ죬�ݴ˷������
��3���Ʊ�����Ȳ�к�������H2S���������ʣ�������ͭ��Һ��ȥ���⣻
��4����Ȳ����̼̼�������ܹ����巢���ӳɷ�Ӧ��
������ǿ���ܹ��Ʊ����ᣬ��Һ©��װ���ᡢ��ƿװ̼���Ρ��Թ�װ��������Һ��������̼���η�Ӧ���ɶ�����̼��������̼��ˮ�����ӷ�Ӧ����̼�����ƣ���A�г���������Ϊ������̼���������ᣬΪ��֤̼�������ǿ�ڱ��ӣ�Ӧ��B���մ��ᣬ�ų�����ԱȽ�̼��ͱ�������ǿ���ĸ��ţ�
��� �⣺��1����ʯ��ˮ��Ӧ������Ȳ���������ƣ���ѧ����ʽ��CaC2+2H2O��C2H2��+Ca��OH��2��
�ʴ�Ϊ��CaC2+2H2O��C2H2��+Ca��OH��2��
��2����ʯ��ˮ��Ӧ�dz����ҡ���Ӧ���ʿ죬ʹ�ñ���ʳ��ˮ���Լ�����ʯ��ˮ��Ӧ�����ʣ�
�ʴ�Ϊ��������ʯ��ˮ��Ӧ�����ʣ�
��3���Ʊ�����Ȳ�к�������H2S���������ʣ�������ͭ��Һ��ȥ���⣻
�ʴ�Ϊ������ͭ��Һ��
��4����Ȳ����̼̼�������ܹ����巢���ӳɷ�Ӧ��ʹ��ˮ��ɫ��
�ʴ�Ϊ����Һ��ɫ��
������ǿ���ܹ��Ʊ����ᣬ��Һ©��װ���ᡢ��ƿװ̼���Ρ��Թ�װ��������Һ��
��1��A�й����Լ���̼���Σ�������̼�����ƣ�
�ʴ�Ϊ��d��
��2���������������̼��ˮ��Ӧ���ɱ��Ӻ�̼�����ƣ���ѧ����ʽ��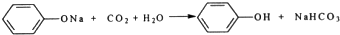 ��
��
�ʴ�Ϊ��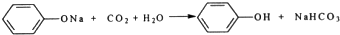 ��
��
��3��Ϊ�ų�����Լ���̼���뱽������ǿ���ĸ��ţ�Bװ���е��Լ�����̼��������Һ��̼����������ᷴӦ���ɴ����ƺͶ�����̼��ˮ�����ӷ���ʽ��CH3COOH+HCO3-=CH3COO-+H2O+CO2�������������ǿ�ڱ��ӣ��������û��Bװ�ã�����Ĵ��ڶԼ���̼���뱽������ǿ����ɸ��ţ����Բ�������
�ʴ�Ϊ������NaHCO3��CH3COOH+HCO3-=CH3COO-+H2O+CO2������������
���� ���⿼������Ȳ��ʵ�����Ʊ������ʵļ��顢���������ǿ����ʵ����ƣ���ȷ�Ʊ�ԭ����ǿ���Ʊ������ԭ���ǽ���ؼ���ע��ʵ����Ƶ������ԣ���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��ˮ�ܱ���������к� | |
| B�� | ��������ֽ� | |
| C�� | ��ˮ����ʹ��̪��Һ��� | |
| D�� | 0��lmol/L��ˮ��Һ������ʱpHԼΪ11 |
| A�� | CaCO3 | B�� | Al��OH��3 | C�� | Mg��OH��2 | D�� | MgCO3 |
| A�� | 1000ml 1mol•L-1�Ȼ�����Һ | B�� | 75 ml 2mol•L-1�Ȼ�����Һ | ||
| C�� | 250 ml 3mol•L-1�Ȼ�����Һ | D�� | 150 ml 1mol•L-1�Ȼ�����Һ |
 ���к��ȵIJⶨ��ʵ�鲽��Ϊ��
���к��ȵIJⶨ��ʵ�鲽��Ϊ��