��Ŀ����
����Ŀ��������(SO2C12)���Ȼ�����(SOC12)�ڿ�������ˮ�����������ҷ�Ӧ������������������������(SO2C12)�������Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��ϳɵķ�ӦʽΪ: SO2(g) + Cl2(g)![]() SO2Cl2(l) ��H=-197.3 kJ��mol-1
SO2Cl2(l) ��H=-197.3 kJ��mol-1
���� | �۵�/�� | �е�/�� | �������� |
SO2C12 | -54.1 | 69.1 | �ֽ���SO2C12 |
�ϳ�SO2C12��װ������ͼ��ʾ(�г�������ʡ�ԣ�����ش��й�����:
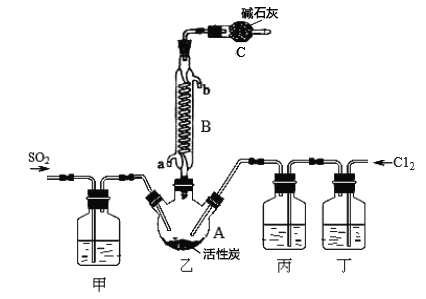
��1��д��Ũ���������ط�Ӧ��ȡCl2�����ӷ���ʽ_________________��
��2������ʢ�ŵ���_____________��
��3����Ӧ���������л������뿪��ʵ����������� _______________��
��4��ͼ��װ��C��������__________________��
��5���Ȼ�����(SOCl2)��ˮ��Ӧ�Ļ�ѧ����ʽΪ_____������A1C13��Һ���ܵõ���ˮAlCl3����SOC12��AlCl3 6H2O�Ļ�ϼ��ȣ��ɵõ���ˮA1C13���Խ���ԭ��__________��
���𰸡�ClO3-+5Cl-+6H+=3Cl2��+3H2O ����ʳ��ˮ ���� ����δ��Ӧ�������Ͷ�������ֹ�����е�ˮ������������ƿ SOCl2+H2O=SO2��+2HCl�� SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ��
��������
��1��~��4����KClO3��Ũ���ᷴӦ�Ƶõ�Cl2�л���HCl��g����H2O��g������װ����Ӧʢ�ű���ʳ��ˮ��ȥCl2�е�HCl��g�����ϳ�SO2Cl2��ԭ��Ϊ��SO2��g��+Cl2��g��![]() SO2Cl2��l����H=-197.3kJ/mol��SO2Cl2��ˮ�����������ҷ�Ӧ������������������SO2��Cl2�ڽ���װ����֮ǰ������װ�üס�����ʢ��Ũ���������ڸ���SO2��Cl2��SO2��Cl2���Ǵ�����Ⱦ�ͬʱSO2Cl2��ˮ�����������ҷ�Ӧ��������������װ��C�м�ʯ�ҵ������ǣ�����δ��Ӧ��SO2��Cl2���Լ���ֹ��������ˮ������������ƿ���ݴ˷�������
SO2Cl2��l����H=-197.3kJ/mol��SO2Cl2��ˮ�����������ҷ�Ӧ������������������SO2��Cl2�ڽ���װ����֮ǰ������װ�üס�����ʢ��Ũ���������ڸ���SO2��Cl2��SO2��Cl2���Ǵ�����Ⱦ�ͬʱSO2Cl2��ˮ�����������ҷ�Ӧ��������������װ��C�м�ʯ�ҵ������ǣ�����δ��Ӧ��SO2��Cl2���Լ���ֹ��������ˮ������������ƿ���ݴ˷�������
��5��SOCl2��ˮ������Ӧ����HCl��SO2��AlCl3��Һ�д���ˮ��ƽ�⣺AlCl3+3H2O![]() Al��OH��3+3HCl������HCl�Ļӷ�������AlCl3��Һʱˮ��ƽ�������ƶ������Ƶ���ˮAlCl3��ʹ��SOCl2��AlCl3��6H2O��ϼ��ȣ�SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ�⣬�ɵ���ˮAlCl3��
Al��OH��3+3HCl������HCl�Ļӷ�������AlCl3��Һʱˮ��ƽ�������ƶ������Ƶ���ˮAlCl3��ʹ��SOCl2��AlCl3��6H2O��ϼ��ȣ�SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ�⣬�ɵ���ˮAlCl3��
��1��Ũ������KClO3��Ӧ����KCl��Cl2��H2O����Ӧ�Ļ�ѧ����ʽΪKClO3+6HCl��Ũ��=KCl+3Cl2��+3H2O�����ӷ���ʽΪClO3-+5Cl-+6H+=3Cl2��+3H2O����Ϊ��ClO3-+5Cl-+6H+=3Cl2��+3H2O
��2��������Ӧ�Ƶõ�Cl2�л���HCl��g����H2O��g���������Լ��������dz�ȥCl2�е�HCl��g��������ʢ�ŵ��DZ���ʳ��ˮ����Ϊ������ʳ��ˮ
��3���������⣬SO2Cl2ͨ������³�Һ̬��SO2Cl2�ķе�ϵͣ��ӷ�����Ӧ������Ӧͨ���������л������뿪������Ϊ������
��4��SO2��Cl2���Ǵ�����Ⱦ�����ֱ���ŷŵ���������SO2Cl2�ڿ�������ˮ�����������ҷ�Ӧ�����������������ͼ��װ��C�������ǣ�����δ��Ӧ��SO2��Cl2����ֹ��������ˮ������������ƿ����Ϊ������δ��Ӧ��SO2��Cl2����ֹ��������ˮ������������ƿ
��5��SOCl2��ˮ�������ҷ�Ӧ��������������˵��SOCl2��H2O��Ӧ����SO2��HCl����Ӧ�Ļ�ѧ����ʽΪSOCl2+H2O=SO2��+2HCl������AlCl3��Һ�д���ˮ��ƽ�⣺AlCl3+3H2O![]() Al��OH��3+3HCl������HCl�Ļӷ�������AlCl3��Һʱˮ��ƽ�������ƶ����ܵõ���ˮAlCl3����SOCl2��AlCl3��6H2O��ϼ��ȿɵõ���ˮAlCl3��ԭ���ǣ�SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ������Ϊ��SOCl2+H2O=SO2��+2HCl�� SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ��
Al��OH��3+3HCl������HCl�Ļӷ�������AlCl3��Һʱˮ��ƽ�������ƶ����ܵõ���ˮAlCl3����SOCl2��AlCl3��6H2O��ϼ��ȿɵõ���ˮAlCl3��ԭ���ǣ�SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ������Ϊ��SOCl2+H2O=SO2��+2HCl�� SOCl2��AlCl3��6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬����SO2��HCl����AlCl3ˮ��

����Ŀ�����±���Ԫ�����ڱ��Ķ����ڣ�����ݱ��е�λ�ã���Ҫ��ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | �� |
(1)��Ԫ�ط�����գ�����ʮ��Ԫ���У���ѧ�������ȶ�����_____��ͨ������»��ϼ�ֻ�и��۶������۵���______��ԭ�Ӱ뾶��С����_______��ԭ�Ӱ뾶��������Ԫ����_________��
(2)����ѧʽ��գ�����ʮ��Ԫ���У�����������ˮ���������ǿ����______��������ǿ��________�������Ե���_________��
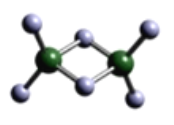
(3)�ٺ͢��γɵ���Ļ�������ṹʽ��_____________��
(4)Ԫ�آ۵���̬�⻯�������ʽ��________________��
(5)�ۿ����γɶ������������һ���Ǻ���ɫ���壬������ѧ����ʽ˵�������岻�˲�����ˮ���ռ���ԭ��__________________________��
����Ŀ���˰�ȫ���������Ⱦ��������������˴���Ĺ㷺���ӡ��ڱ�ը�ĺ˵�վ��Χ���з��������ʵ�һ131���һ 137���⡪131�������������룬���ܻ�������״�ٵȼ�����
(l)Cs(�)�ļ۵��ӵĵ����Ų�ʽΪ6s1�����ͬ�����ǰ�����ڣ������������ڣ�������Ԫ��X��Y��Z�ĵ��������±���
Ԫ�ش��� | X | Y | Z |
��һ�����ܣ�kJ��mol-1) | 520 | 496 | 419 |
��������Ԫ��X��Y��Z��Ԫ�ط��ŷֱ�Ϊ_________����̬Zԭ�ӵĺ�������Ų�ʽΪ______��X�γɵĵ��ʾ����к��еĻ�ѧ��������_________________��
(2)F��Iͬ���壬BeF2��H2O����������ԭ�ӹ��ɵĹ��ۻ�������ӣ����߷����е�����ԭ��Be��O���ӻ���ʽ�ֱ�Ϊ______��______��BeF2���ӵ����幹����____________��H2O���ӵ����幹����________________��
(3)���ͬ������Ⱦ��к�ǿ�Ļ����ԣ����γɴ����ĺ��Ȼ����BC13������B��C1���ļ���Ϊ__________________��
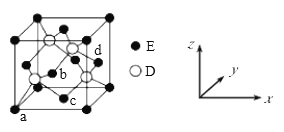
(4)131I2����ľ����ṹ��ͼ����ʾ���þ����к���____��131I2���ӣ�KI�ľ����ṹ��ͼ����ʾ��ÿ��K+����______��I-��

(5)KI������ܶ�Ϊ��g cm 3��K��I��Ħ�������ֱ�ΪMK g mol-1��M1g mol-1��ԭ�Ӱ뾶�ֱ�ΪrKpm��r1 pm�������ӵ�����ֵΪNA����KI������ԭ�ӵ����ռ��������İٷ���Ϊ_____________��