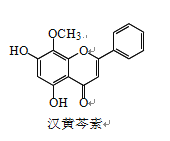
��Ŀ����
����Ŀ����t��ʱ����agNH3��ȫ����ˮ���õ�V mL��Һ���������Һ���ܶ�Ϊ��g/cm-3����������Ϊ�أ����к�NH4+�����ʵ���Ϊb moL��������������ȷ���ǣ�������
A. ���ʵ����ʵ���Ũ��c=1000a/35Vmol/L
B. ���ʵ���������Ϊ��=a /(V��-a)��100%
C. ��Һ��c��OH-��=1000b/Vmol/L+c��H+��
D. ������Һ���ټ���VmLˮ��������Һ��������������0.5��
���𰸡�C
��������
A.a g NH3�����ʵ���Ϊ![]() =
=![]() mol����Һ���ΪVmL��������Һ�����ʵ���Ũ��Ϊ
mol����Һ���ΪVmL��������Һ�����ʵ���Ũ��Ϊ![]() =
=![]() mol/L����A����
mol/L����A����
B.��ˮ��Һ����Ϊ����������Һ���ܶ�Ϊ�� gcm-3�����ΪVmL��������Һ����Ϊ��Vg�����ʰ���������Ϊag�����ʵ���������Ϊ![]() ��100%=
��100%=![]() ��100%����B����
��100%����B����
C.��ҺOH-��Դ��һˮ�ϰ���ˮ�ĵ��룬NH4+��Ũ��Ϊ![]() =
=![]() mol/L��һˮ�ϰ�����NH3H2O
mol/L��һˮ�ϰ�����NH3H2O![]() NH4++OH-������Һ�еĵ���غ��֪��c��OH-��=c��NH4+��+c��H+��=1000b/Vmol/L+c��H+������C��ȷ��
NH4++OH-������Һ�еĵ���غ��֪��c��OH-��=c��NH4+��+c��H+��=1000b/Vmol/L+c��H+������C��ȷ��
D.ˮ���ܶȱȰ�ˮ���ܶȴ��������İ�ˮ��ˮ��ˮ�������������Ϻ���Һ����������ԭ��ˮ��2������Һ�а�����������ͬ����������������Һ���ʵ���������С��0��5w����D����
��ѡC��

����Ŀ��������(SO2C12)���Ȼ�����(SOC12)�ڿ�������ˮ�����������ҷ�Ӧ������������������������(SO2C12)�������Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��ϳɵķ�ӦʽΪ: SO2(g) + Cl2(g)![]() SO2Cl2(l) ��H=-197.3 kJ��mol-1
SO2Cl2(l) ��H=-197.3 kJ��mol-1
���� | �۵�/�� | �е�/�� | �������� |
SO2C12 | -54.1 | 69.1 | �ֽ���SO2C12 |
�ϳ�SO2C12��װ������ͼ��ʾ(�г�������ʡ�ԣ�����ش��й�����:
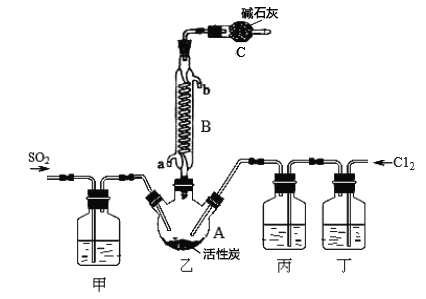
��1��д��Ũ���������ط�Ӧ��ȡCl2�����ӷ���ʽ_________________��
��2������ʢ�ŵ���_____________��
��3����Ӧ���������л������뿪��ʵ����������� _______________��
��4��ͼ��װ��C��������__________________��
��5���Ȼ�����(SOCl2)��ˮ��Ӧ�Ļ�ѧ����ʽΪ_____������A1C13��Һ���ܵõ���ˮAlCl3����SOC12��AlCl3 6H2O�Ļ�ϼ��ȣ��ɵõ���ˮA1C13���Խ���ԭ��__________��
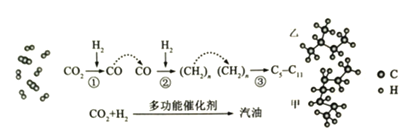
����Ŀ������һ����Ҫ�Ĺ��ɽ���Ԫ�أ�ͨ�������Ͻ���ֵ����Ӽ�������ǿ�Ͻ��ǿ�ȡ�Ӳ�ȡ��ɺ��Եȡ������ƾ���(Na2MoO4��2H2O)����Ϊ��������ȴˮϵͳ�Ľ�����ʴ���Ƽ�����ͼ15�ǻ����������Ի����(��Ҫ�ɷ�Ϊ����MoS2)Ϊԭ�����Ʊ������⡢�����ƾ������Ҫ����ͼ��
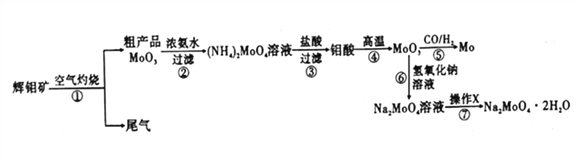
�ش���������:
��1����֪��Ӧ��Ϊ���ֽⷴӦ����������Կ�Ļ��ϼ�Ϊ___________��
��2����Ӧ�������ӷ���ʽΪ___________��
��3�����������ʱ�Ļ�ѧ����ʽΪ____________��
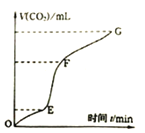
��4������XΪ_________����֪��������һ���¶ȷ�Χ�ڵ��������ʼ���Ӧ���ʵ��ܽ�����±���ʾ�����ڲ���X��Ӧ�����¶ȵ���ѷ�ΧΪ_______(�����)��
�¶�(��) | 0 | 4 | 9 | 10 | 15.5 | 32 | 51.5 | 100 | >100 |
�������� | Na2MoO4��10H2O | Na2MoO4��2H2O | Na2MoO4 | ||||||
�ܽ�� | 30.63 | 33.85 | 38.16 | 39.28 | 39.27 | 39.82 | 41.27 | 45.57 | |
A.0�桫10�� B.10�桫100�� C.15.5�桫50�� D.100������
��5���Ʊ������ƾ��廹����ͨ�����ƵĻ������ֱ�Ӽ������������Һ�����ķ����������������У��������������ɣ����������뻹ԭ�������ʵ���֮��Ϊ_________��
��6��Li��MoS2�ɳ���صĹ���ԭ��ΪxLi+nMoS2![]() Lix(MoS2)n[Lix(MoS2)n�����ڵ缫��]�����س��ʱ�����ĵ缫��ӦʽΪ___________________��
Lix(MoS2)n[Lix(MoS2)n�����ڵ缫��]�����س��ʱ�����ĵ缫��ӦʽΪ___________________��
��7�����û�ԭ������(CO��H2)��ԭMoO3���⣬ҵ���Ʊ���ԭ������CO��H2�ķ�Ӧԭ��֮һΪCO2+CH4![]() 2CO+2H2���������������Ϊ90%��7L(��״��)��Ȼ��������������̼�ڸ����·�Ӧ������ת����Ϊ80%,�ò�����CO��H2��ԭMoO3���⣬�������������������Ϊ_________��
2CO+2H2���������������Ϊ90%��7L(��״��)��Ȼ��������������̼�ڸ����·�Ӧ������ת����Ϊ80%,�ò�����CO��H2��ԭMoO3���⣬�������������������Ϊ_________��