题目内容
(14分)1773年,伊莱尔·罗埃尔(Hilaire Rouelle)发现尿素。1828年,弗里德里希·维勒首次使用无机物质氰酸钾[KCNO]与硫酸铵人工合成了尿素[CO(NH2)2]。
(1)维勒合成尿素的化学反应方程式为 。
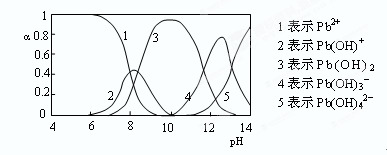
(2)工业上尿素是由CO2和NH3在一定条件下合成,其反应方程式为 当氨碳比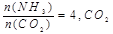 的转化率随时间的变化关系如图1所示。
的转化率随时间的变化关系如图1所示。
①A点速率v逆(CO2)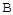 点速率为V正(CO2)(填“大于”、“小于”或“等于”)
点速率为V正(CO2)(填“大于”、“小于”或“等于”)
②NH3的平衡转化率为 。
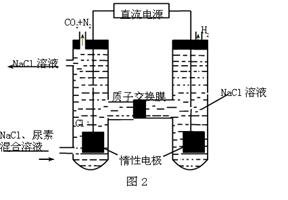
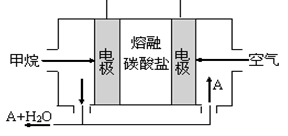
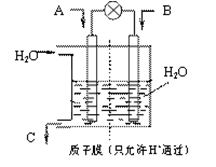
(3)人工肾脏可用间接电化学方法除去代谢产物中的尿素,原理如图2。
①电源的负极为 (填“A”或“B”)。
②阴极室中发生的电极反应式为
③电解结束后,阴极室溶液的pH与电解前相比将 (填“增大”、“减小”、“不变”)若两极共收集到气体11.2L(标准状况),则除去的尿素为 g(忽略气体的溶解)。
(1)维勒合成尿素的化学反应方程式为 。
(2)工业上尿素是由CO2和NH3在一定条件下合成,其反应方程式为 当氨碳比
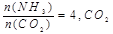 的转化率随时间的变化关系如图1所示。
的转化率随时间的变化关系如图1所示。①A点速率v逆(CO2)
 点速率为V正(CO2)(填“大于”、“小于”或“等于”)
点速率为V正(CO2)(填“大于”、“小于”或“等于”)②NH3的平衡转化率为 。


(3)人工肾脏可用间接电化学方法除去代谢产物中的尿素,原理如图2。
①电源的负极为 (填“A”或“B”)。
②阴极室中发生的电极反应式为
③电解结束后,阴极室溶液的pH与电解前相比将 (填“增大”、“减小”、“不变”)若两极共收集到气体11.2L(标准状况),则除去的尿素为 g(忽略气体的溶解)。
(14分)
(1)2KCNO+(NH4)2SO4=2CO(NH2)2+K2SO4
(2)2NH3+CO2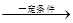 CO(NH2)2+H2O 小于 30%
CO(NH2)2+H2O 小于 30%
(3)B (1分) 2H++2e- ="=" H2 ↑ (1分) 不变 6.0 (其余均2分)
(1)2KCNO+(NH4)2SO4=2CO(NH2)2+K2SO4
(2)2NH3+CO2
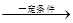 CO(NH2)2+H2O 小于 30%
CO(NH2)2+H2O 小于 30%(3)B (1分) 2H++2e- ="=" H2 ↑ (1分) 不变 6.0 (其余均2分)
(1)一个简单的复分解反应:2KCNO+(NH4)2SO4=2CO(NH2)2+K2SO4
(2)根据反应物和生成物可知,反应的方程式是2NH3+CO2 CO(NH2)2+H2O。
CO(NH2)2+H2O。
①根据图像可知,B点CO2的转化率不再发生变化,所以是平衡状态。因此A点没有达到平衡状态,因此A点的逆反应速率小于B的点的正反应速率;
②如果设CO2的物质的量是1mol,则氨气是4mol。根据图像可知,CO2的转化率是0.6,则消耗CO2是0.6mol,所以消耗氨气是1.2mol,因此氨气的转化率是1.2÷4=0.3,即30%。
(3)①根据装置图可知,和电源B极相连的产生氢气,所以该电极是阴极,氢离子放电,所以B是电源的负极,A是正极。
②阳极失去电子,所以溶液中的氯离子放电,生成氯气。氯气具有氧化性,能氧化尿素生成氮气和CO2,方程式分别是2Cl--2e-=Cl2↑、CO(NH2)2+3Cl2+H2O=N2+CO2+6HCl。
③由以上的方程式可计算出除去的尿素为6.0g
(2)根据反应物和生成物可知,反应的方程式是2NH3+CO2
 CO(NH2)2+H2O。
CO(NH2)2+H2O。①根据图像可知,B点CO2的转化率不再发生变化,所以是平衡状态。因此A点没有达到平衡状态,因此A点的逆反应速率小于B的点的正反应速率;
②如果设CO2的物质的量是1mol,则氨气是4mol。根据图像可知,CO2的转化率是0.6,则消耗CO2是0.6mol,所以消耗氨气是1.2mol,因此氨气的转化率是1.2÷4=0.3,即30%。
(3)①根据装置图可知,和电源B极相连的产生氢气,所以该电极是阴极,氢离子放电,所以B是电源的负极,A是正极。
②阳极失去电子,所以溶液中的氯离子放电,生成氯气。氯气具有氧化性,能氧化尿素生成氮气和CO2,方程式分别是2Cl--2e-=Cl2↑、CO(NH2)2+3Cl2+H2O=N2+CO2+6HCl。
③由以上的方程式可计算出除去的尿素为6.0g

练习册系列答案
 永乾教育寒假作业快乐假期延边人民出版社系列答案
永乾教育寒假作业快乐假期延边人民出版社系列答案
相关题目
 CH3OH(g) ΔH
CH3OH(g) ΔH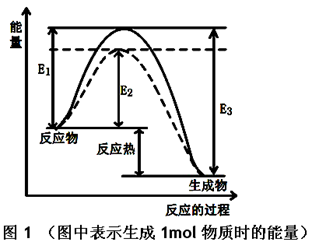

 H2O(l) 的ΔH =" 40.69" kJ·mol-1
H2O(l) 的ΔH =" 40.69" kJ·mol-1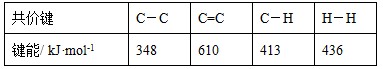
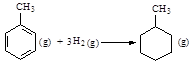 的ΔH为-384 kJ·mol-1
的ΔH为-384 kJ·mol-1
 N2O4(g) △H <0的平衡常数 K=13.3,则该条件下密闭容器中N2O4和NO2的混合气体达到平衡时,若 c (NO2) =" 0.0300" mol·L-1,
N2O4(g) △H <0的平衡常数 K=13.3,则该条件下密闭容器中N2O4和NO2的混合气体达到平衡时,若 c (NO2) =" 0.0300" mol·L-1, 2OH?+H2↑+Cl2↑
2OH?+H2↑+Cl2↑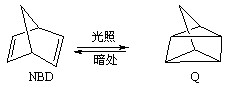 ,利用该反应可以贮存太阳能,则NBD的内能比Q的内能高
,利用该反应可以贮存太阳能,则NBD的内能比Q的内能高 2NH3(g)+ 3/2O2(g);△H=+630kJ·mol-1
2NH3(g)+ 3/2O2(g);△H=+630kJ·mol-1 CO2+NO △H =" a" kJ/mol达到平衡后,降低温度,混和气体的颜色变浅。下列判断正确的是
CO2+NO △H =" a" kJ/mol达到平衡后,降低温度,混和气体的颜色变浅。下列判断正确的是