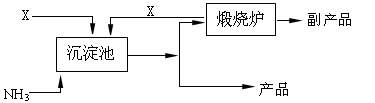
��Ŀ����
��13�֣��ɷ�Ǧ��PbS���Ʊ�Pb��PbO2�ķ������£�
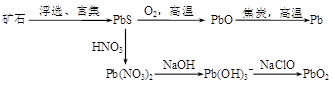
PbO�뽹̿����ʱ���ܻᷢ�����·�Ӧ��
PbO(s)��C(s)��Pb(s)��CO(g) ��H����108.5 kJ��mol��1 ��
PbO(s)��CO(g)��Pb(s)��CO2(g) ��H����64 kJ��mol��1 ��
��1����֪Pb��O2��Ӧ���Ȼ�ѧ����ʽΪ��2Pb(s)��O2(g)��2PbO(s) ��H����438 kJ��mol��1
��C��ȫȼ�յ��Ȼ�ѧ����ʽΪ ��
��2������߷�Ӧ����PbOת���ʵĴ�ʩ�� ������ĸ����
a�������¶� b������ѹǿ c�����뽹̿ d���������
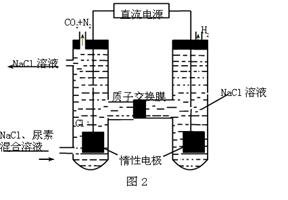
��3���Ʊ������л����SO2��NO��CO���ж����壬�ɽ����ǰ�һ�����������һ�������·�Ӧ�õ�S��N2��CO2����SO2��NO�������Ϊ1��2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ��Һ��Ǧ�Ĵ�����̬�ж��֣�����̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��
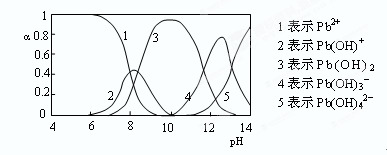
pH��6.5ʱ��Pb2��ˮ������ӷ���ʽΪ ������NaClO�Ʊ�PbO2֮ǰ������NaOH������ҺpH��12.5�������� ��
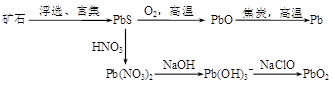
PbO�뽹̿����ʱ���ܻᷢ�����·�Ӧ��
PbO(s)��C(s)��Pb(s)��CO(g) ��H����108.5 kJ��mol��1 ��
PbO(s)��CO(g)��Pb(s)��CO2(g) ��H����64 kJ��mol��1 ��
��1����֪Pb��O2��Ӧ���Ȼ�ѧ����ʽΪ��2Pb(s)��O2(g)��2PbO(s) ��H����438 kJ��mol��1
��C��ȫȼ�յ��Ȼ�ѧ����ʽΪ ��
��2������߷�Ӧ����PbOת���ʵĴ�ʩ�� ������ĸ����
a�������¶� b������ѹǿ c�����뽹̿ d���������
��3���Ʊ������л����SO2��NO��CO���ж����壬�ɽ����ǰ�һ�����������һ�������·�Ӧ�õ�S��N2��CO2����SO2��NO�������Ϊ1��2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ��Һ��Ǧ�Ĵ�����̬�ж��֣�����̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��

pH��6.5ʱ��Pb2��ˮ������ӷ���ʽΪ ������NaClO�Ʊ�PbO2֮ǰ������NaOH������ҺpH��12.5�������� ��
��1��C(s)��O2(g)��CO2(g) ��H����393.5 kJ��mol��1 ��2��c
��3��SO2��2NO��4CO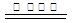 S��N2��4CO2
S��N2��4CO2
��4��Pb2����H2O Pb(OH)����H�� ��ʱPb(OH)3���������
Pb(OH)����H�� ��ʱPb(OH)3���������
���ڣ�4����ÿ��2�֣�����ÿ��3�֣���13�֣�
��3��SO2��2NO��4CO
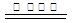 S��N2��4CO2
S��N2��4CO2��4��Pb2����H2O
 Pb(OH)����H�� ��ʱPb(OH)3���������
Pb(OH)����H�� ��ʱPb(OH)3������������ڣ�4����ÿ��2�֣�����ÿ��3�֣���13�֣�
��1�����ݸ�˹���ɿ�֪���٣��ڣ��ۼ��õ�C(s)��O2(g)��CO2(g) �����Է�Ӧ�Ȧ�H����108.5 kJ��mol��1�D64 kJ��mol��1�D438 kJ��mol��1����393.5 kJ��mol��1��
��2������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���ת���ʽ��ͣ���Ӧǰ��������䣬�ı�ѹǿƽ�ⲻ�ƶ�����������Ӱ��ƽ��״̬�����뽹̿��������CO2������CO2Ũ�ȣ�ƽ��������Ӧ�����ƶ���ת��������ѡc��
��3�����ݻ��ϼ۵ı仯��֪��SO2��CO�ǻ�ԭ����NO�������������Ը��ݵ��ӵĵ�ʧ�غ��֪����Ӧ�ķ���ʽ��SO2��2NO��4CO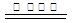 S��N2��4CO2��
S��N2��4CO2��
��4������ͼ���֪��pH��6.5ʱ��ˮ�������Pb(OH)��������ˮ�ⷽ��ʽ��Pb2����H2O Pb(OH)����H��������ͼ���֪����ʱ��Һ��Pb(OH)3��������ߣ���Ӧ���ڷ�����
Pb(OH)����H��������ͼ���֪����ʱ��Һ��Pb(OH)3��������ߣ���Ӧ���ڷ�����
��2������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���ת���ʽ��ͣ���Ӧǰ��������䣬�ı�ѹǿƽ�ⲻ�ƶ�����������Ӱ��ƽ��״̬�����뽹̿��������CO2������CO2Ũ�ȣ�ƽ��������Ӧ�����ƶ���ת��������ѡc��
��3�����ݻ��ϼ۵ı仯��֪��SO2��CO�ǻ�ԭ����NO�������������Ը��ݵ��ӵĵ�ʧ�غ��֪����Ӧ�ķ���ʽ��SO2��2NO��4CO
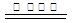 S��N2��4CO2��
S��N2��4CO2����4������ͼ���֪��pH��6.5ʱ��ˮ�������Pb(OH)��������ˮ�ⷽ��ʽ��Pb2����H2O
 Pb(OH)����H��������ͼ���֪����ʱ��Һ��Pb(OH)3��������ߣ���Ӧ���ڷ�����
Pb(OH)����H��������ͼ���֪����ʱ��Һ��Pb(OH)3��������ߣ���Ӧ���ڷ�����
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2NH3��g����H��0�ﵽƽ��������¶ȣ���Ӧ����v(H2)��H2��ƽ��ת���ʾ�����
2NH3��g����H��0�ﵽƽ��������¶ȣ���Ӧ����v(H2)��H2��ƽ��ת���ʾ����� 2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����
2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ����� 1/2N2O4��g�� ��H =��26��35 kJ��mol��1
1/2N2O4��g�� ��H =��26��35 kJ��mol��1
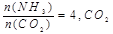 ��ת������ʱ��ı仯��ϵ��ͼ1��ʾ��
��ת������ʱ��ı仯��ϵ��ͼ1��ʾ��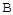 ������ΪV����CO2��������ڡ�����С�ڡ����ڡ���
������ΪV����CO2��������ڡ�����С�ڡ����ڡ���
 H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���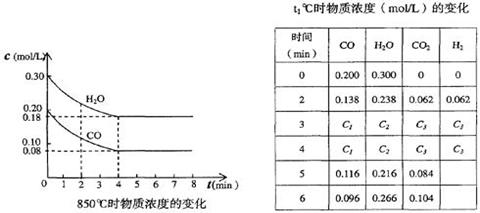
 1/2N2O4(g) ��H = ��26.35 kJ��mol��1
1/2N2O4(g) ��H = ��26.35 kJ��mol��1 CH3OH(g) ��H1
CH3OH(g) ��H1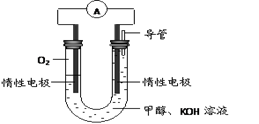
 2NH3 �ں����ܱ������н��У��ﵽƽ��״̬�ı�־��
2NH3 �ں����ܱ������н��У��ﵽƽ��״̬�ı�־��