��Ŀ����
��ҵ��һ���ں����ܱ������п��Բ������з�Ӧ�ϳɼ״���
CO��g��+2H2��g�� CH3OH��g�� ��H
CH3OH��g�� ��H
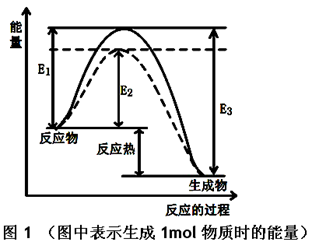

��1������ͼ1��д���ϳɼ״����Ȼ�ѧ����ʽ
��������E1��E2��E3��ʾ����
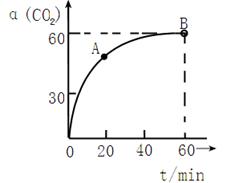
��2���÷�Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ����ͼ2��t1ʱ�ı���ij���������ı������������ ��
��3���жϷ�Ӧ�ﵽƽ��״̬�������� (����ĸ��ţ���ͬ)��
E��������CO��H2��CH3OH��Ũ��֮��Ϊ1:2:1
��4����һ���¶��£�����4a mol H2��2amol CO����2L���ܱ������У���ַ�Ӧ����CO��ת����Ϊ50������÷�Ӧ��ƽ�ⳣ��Ϊ ������ʱ�����������Ͷ��a mol CO��2amol H2��amol CH3OH���ж�ƽ���ƶ��ķ����� ���������ƶ����������ƶ������ƶ���������ԭƽ����ȣ�CO�����ʵ���Ũ�� ������������䡱��С������
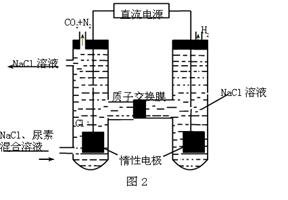
��5��ij����ȼ�ϵ����������̼����Ϊ����ʣ�CH4Ϊȼ�ϣ�����Ϊ��������ϡ�������������缫��Ϊ��ʹ��ȼ�ϵ�س�ʱ���ȶ����У���صĵ�������Ӧ�����ȶ�����ع���ʱ�����в���A���ʲμ�ѭ��������ͼ����A���ʵĻ�ѧʽ��_________����ԭ��صĸ�����Ӧʽ�ɱ�ʾΪ ��
CO��g��+2H2��g��
 CH3OH��g�� ��H
CH3OH��g�� ��H

��1������ͼ1��д���ϳɼ״����Ȼ�ѧ����ʽ
��������E1��E2��E3��ʾ����
��2���÷�Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ����ͼ2��t1ʱ�ı���ij���������ı������������ ��
��3���жϷ�Ӧ�ﵽƽ��״̬�������� (����ĸ��ţ���ͬ)��
| A��2v(H2)(��) =v(CO)(��) |
| B�����������ܶȲ��� |
| C����������ƽ����Է����������� |
| D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯 |
��4����һ���¶��£�����4a mol H2��2amol CO����2L���ܱ������У���ַ�Ӧ����CO��ת����Ϊ50������÷�Ӧ��ƽ�ⳣ��Ϊ ������ʱ�����������Ͷ��a mol CO��2amol H2��amol CH3OH���ж�ƽ���ƶ��ķ����� ���������ƶ����������ƶ������ƶ���������ԭƽ����ȣ�CO�����ʵ���Ũ�� ������������䡱��С������
��5��ij����ȼ�ϵ����������̼����Ϊ����ʣ�CH4Ϊȼ�ϣ�����Ϊ��������ϡ�������������缫��Ϊ��ʹ��ȼ�ϵ�س�ʱ���ȶ����У���صĵ�������Ӧ�����ȶ�����ع���ʱ�����в���A���ʲμ�ѭ��������ͼ����A���ʵĻ�ѧʽ��_________����ԭ��صĸ�����Ӧʽ�ɱ�ʾΪ ��

��1��CO(g) + 2H2(g)
 CH3OH(g) ��H=��E1��E3����E3��E1��kJ?mol-1
CH3OH(g) ��H=��E1��E3����E3��E1��kJ?mol-1��2�֣�ûд����״̬�����֣���д��λ���۷֣�
��2����ѹ���ȱ����ӷ�Ӧ�����������Ӽ״�Ũ�ȡ������¶ȡ�ʹ�ô����ȣ�2�֣����δ�һ�������𰸸��֡�ע��t1ʱ�̲���ȷ���Ƿ�ƽ�⣩
��3��CD��2�֣����������֣�
��4��
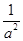 ��2�֣������ƶ���2�֣�����1�֣�
��2�֣������ƶ���2�֣�����1�֣���5��AΪCO2��2�֣�CH4+4CO32-��8e- =5CO2+2H2O ��2�֣�
�����������1����ͼ�п�֪��E1Ϊ���ѻ�ѧ������Ҫ��������E3Ϊ�γɻ�ѧ��ʱ���ͷŵ���������ÿ����1mol�״��ķ�Ӧ��Ϊ��E1��E3��KJ/mol��
��2����ͼ2�����Ͽ��Կ�����t1ʱ��ǰ��t1ʱ�̺��淴Ӧ����˲������ʹ��Ӧ�����������������ѹ�����¡��Ӵ�������������Ũ�ȡ�
��3���жϷ�Ӧ�Ƿ�ﵽƽ������������淴Ӧ������Ⱥ�����Ũ�ȱ��ֲ��䡣�ڸ÷�Ӧ�У�
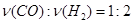 ����
����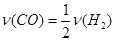 ����A���˵����Ӧ�Ѵﵽƽ��״̬���÷�Ӧ�ں��������н��У���Ӧ�������ﶼ����̬����������������������һֱ���䣬�ܶ�Ҳһֱ���䣬���B��Ҳ����˵����Ӧ�ﵽƽ��״̬�������ʵ������Կ�Ϊ��λʱ��ƽ����Է�����������ֵ�ϵ���ƽ��Ħ��������
����A���˵����Ӧ�Ѵﵽƽ��״̬���÷�Ӧ�ں��������н��У���Ӧ�������ﶼ����̬����������������������һֱ���䣬�ܶ�Ҳһֱ���䣬���B��Ҳ����˵����Ӧ�ﵽƽ��״̬�������ʵ������Կ�Ϊ��λʱ��ƽ����Է�����������ֵ�ϵ���ƽ��Ħ��������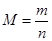 ����Ϊ�÷�Ӧ��һ�����������Ŀ�����仯�ķ�Ӧ����������ʵ���Ҳ�ᷢ���仯��ֻ�е���Ӧ�ﵽƽ�����������ʵ����Ų��ٱ仯����ƽ����Է����������ٱ仯�����C���˵����Ӧ�ﵽƽ��״̬��D�������Ũ�Ȳ��ٱ仯����Ӧ�ﵽƽ��״̬����Ӧ�ﵽƽ��ʱ�������ʵİٷֺ���һ�������ʼ�ı���һ��������ֵ��һ����1:2:1��E���˵����Ӧ�ﵽƽ��״̬��
����Ϊ�÷�Ӧ��һ�����������Ŀ�����仯�ķ�Ӧ����������ʵ���Ҳ�ᷢ���仯��ֻ�е���Ӧ�ﵽƽ�����������ʵ����Ų��ٱ仯����ƽ����Է����������ٱ仯�����C���˵����Ӧ�ﵽƽ��״̬��D�������Ũ�Ȳ��ٱ仯����Ӧ�ﵽƽ��״̬����Ӧ�ﵽƽ��ʱ�������ʵİٷֺ���һ�������ʼ�ı���һ��������ֵ��һ����1:2:1��E���˵����Ӧ�ﵽƽ��״̬����4����Ӧ����CO�����ʵ�����2a��50%=amol��
CO(g)+2H2(g)
 CH3OH(g)
CH3OH(g)��ʼ����2amol 4amol 0
�仯����amol 2amol amol
ƽ������amol 2amol amol
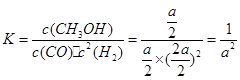
�������������Ͷ��a mol CO��2amol H2��amol CH3OH���൱����������ѹǿ��ƽ�������ƶ�����ԭƽ����ȣ���Ϊ������CO�������������ݻ�δ�䣬����CO��Ũ������
��5���õ��Ϊ����ȼ�ϵ�أ�����ܷ�ӦΪCH4+2O2=CO2+2H2O����صĵ����Ϊ̼���Σ��ҵ������Һ��ɱ����ȶ�������ѭ��������ӦΪCO2��ȼ�ϵ�ظ���Ӧͨ��ȼ�ϣ��ʸ�����ӦʽΪ��CH4+4CO32-��8e- =5CO2+2H2O��

��ϰ��ϵ�д�
�����Ŀ
 [Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)3]Ac��CO ��H��0

 CH(g)��H2(g) ��H1="156.6" kJ/mol
CH(g)��H2(g) ��H1="156.6" kJ/mol
 CH2(g)=CH4(g)��HC
CH2(g)=CH4(g)��HC
 CH(g ) ��H2="32.4" kJ/mol
CH(g ) ��H2="32.4" kJ/mol CH2(g)��H2(g)�ġ�H= kJ/mol��
CH2(g)��H2(g)�ġ�H= kJ/mol�� HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��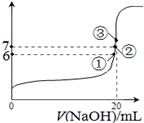
 SO2Cl2(l) ��H���C97.3kJ��mol��1
SO2Cl2(l) ��H���C97.3kJ��mol��1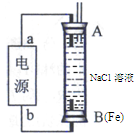
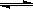 CO2(g) + H2(g)���õ������������ݣ�
CO2(g) + H2(g)���õ������������ݣ� 2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����
2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����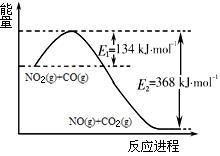
 2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
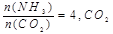 ��ת������ʱ��ı仯��ϵ��ͼ1��ʾ��
��ת������ʱ��ı仯��ϵ��ͼ1��ʾ�� ������ΪV����CO2��������ڡ�����С�ڡ����ڡ���
������ΪV����CO2��������ڡ�����С�ڡ����ڡ���