��Ŀ����
CO2��SO2��NOx �ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx �ǽ������ЧӦ����������⻯ѧ��������Ч;����
��1�����д�ʩ�У������ڽ��ʹ����е�CO2��SO2��NOx Ũ�ȵ��� ������ĸ��
a�����ٻ�ʯȼ�ϵ�ʹ�ã���������Դ
b��ʹ�������䣬���ٷ��ﰺ�ŷ�
c���ಽ�л�˹�����������ר����˽�ҳ�
d������ҵ�����ü�Һ���պ����ŷ�
��2��Ϊ�˽�������β���Դ�������Ⱦ���йز������ü״������Ϊ��������ȼ�ϡ�д���úϳ���(CO��H2)�����״��Ļ�ѧ����ʽ ����֪�÷�Ӧ�ϳ�1 molҺ̬�״���������131.9 kJ�� 2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1����д��Һ̬�״�ȼ�����ɶ�����̼��ˮ�������Ȼ�ѧ����ʽ ��
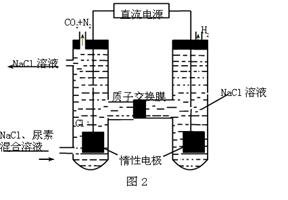
��3��������������ͼ��ʾװ���õ绯ѧԭ����CO2��SO2ת��Ϊ��Ҫ����ԭ�ϡ�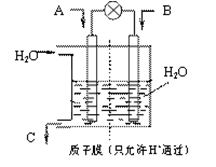
��AΪCO2��BΪH2��CΪCH3OH����ͨ��CO2��һ��Ϊ ������AΪSO2��BΪO2��CΪH2SO4�����ĵ缫��ӦʽΪ ��
��4�������о������������ת��ʱ��ijС����ĵ��������ݣ�17�桢1.01��105 Paʱ��
2NO2(g)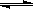 N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
c (N2O4)�� ��������λ��Ч���֣���
�ڸı�������ϵ��ij���������ﵽ�µ�ƽ���û�������� c (NO2) =" 0.04" mol��L��1�� c (N2O4) =" 0.007" mol��L��1����ı������Ϊ ��
��1�����д�ʩ�У������ڽ��ʹ����е�CO2��SO2��NOx Ũ�ȵ��� ������ĸ��
a�����ٻ�ʯȼ�ϵ�ʹ�ã���������Դ
b��ʹ�������䣬���ٷ��ﰺ�ŷ�
c���ಽ�л�˹�����������ר����˽�ҳ�
d������ҵ�����ü�Һ���պ����ŷ�
��2��Ϊ�˽�������β���Դ�������Ⱦ���йز������ü״������Ϊ��������ȼ�ϡ�д���úϳ���(CO��H2)�����״��Ļ�ѧ����ʽ ����֪�÷�Ӧ�ϳ�1 molҺ̬�״���������131.9 kJ�� 2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1����д��Һ̬�״�ȼ�����ɶ�����̼��ˮ�������Ȼ�ѧ����ʽ ��
��3��������������ͼ��ʾװ���õ绯ѧԭ����CO2��SO2ת��Ϊ��Ҫ����ԭ�ϡ�

��AΪCO2��BΪH2��CΪCH3OH����ͨ��CO2��һ��Ϊ ������AΪSO2��BΪO2��CΪH2SO4�����ĵ缫��ӦʽΪ ��
��4�������о������������ת��ʱ��ijС����ĵ��������ݣ�17�桢1.01��105 Paʱ��
2NO2(g)
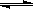 N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��c (N2O4)�� ��������λ��Ч���֣���
�ڸı�������ϵ��ij���������ﵽ�µ�ƽ���û�������� c (NO2) =" 0.04" mol��L��1�� c (N2O4) =" 0.007" mol��L��1����ı������Ϊ ��
��1��acd ��2�֣�
��2��CO��2 H2��CH3OH��2�֣�
2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g) ��H =��1452 kJ��mol��1��3�֣�
��3������1�֣��� SO2��2H 2O��2e�� = SO42���� 4H+ ��2�֣���
��4����0.012 mol��L��1 ��2�֣��������¶ȣ�2�֣�
��2��CO��2 H2��CH3OH��2�֣�
2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g) ��H =��1452 kJ��mol��1��3�֣�
��3������1�֣��� SO2��2H 2O��2e�� = SO42���� 4H+ ��2�֣���
��4����0.012 mol��L��1 ��2�֣��������¶ȣ�2�֣�
��1��ʹ�������䣬���ٷ��ﰺ�ŷţ������Ƴ������ƻ��ģ�b����ȷ���������ȷ�ģ���ѡacd��
��2��������֪�ķ�Ӧ����������֪������ʽΪCO��2H2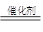 CH3OH����Ӧ��CO(g)��2H2(g)
CH3OH����Ӧ��CO(g)��2H2(g)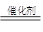 CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1��
CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1��
��3���ڷ�Ӧ��̼Ԫ�صĻ��ϼ۽��ͣ�����CO2�����������õ����ӣ����CO2������ͨ�롣SO2ʧȥ���ӣ��ڸ���ͨ�룬�缫��ӦʽΪSO2��2H 2O��2e�� = SO42���� 4H+ ��
��4���ٸ���ƽ�ⳣ������ʽ��֪��c (N2O4)��13.3��0.03002��0.012 mol��L��1��
�ڴ�ʱƽ�ⳣ����0.007��0.042��4.375����ƽ�ⳣ����С����������Ӧ�Ƿ��ȷ�Ӧ�����Ըı�������������¶ȣ�ƽ�����淴Ӧ�����ƶ���
��2��������֪�ķ�Ӧ����������֪������ʽΪCO��2H2
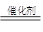 CH3OH����Ӧ��CO(g)��2H2(g)
CH3OH����Ӧ��CO(g)��2H2(g)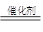 CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1��
CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1����3���ڷ�Ӧ��̼Ԫ�صĻ��ϼ۽��ͣ�����CO2�����������õ����ӣ����CO2������ͨ�롣SO2ʧȥ���ӣ��ڸ���ͨ�룬�缫��ӦʽΪSO2��2H 2O��2e�� = SO42���� 4H+ ��
��4���ٸ���ƽ�ⳣ������ʽ��֪��c (N2O4)��13.3��0.03002��0.012 mol��L��1��
�ڴ�ʱƽ�ⳣ����0.007��0.042��4.375����ƽ�ⳣ����С����������Ӧ�Ƿ��ȷ�Ӧ�����Ըı�������������¶ȣ�ƽ�����淴Ӧ�����ƶ���

��ϰ��ϵ�д�
�����Ŀ

 CH(g)��H2(g) ��H1="156.6" kJ/mol
CH(g)��H2(g) ��H1="156.6" kJ/mol
 CH2(g)=CH4(g)��HC
CH2(g)=CH4(g)��HC
 CH(g ) ��H2="32.4" kJ/mol
CH(g ) ��H2="32.4" kJ/mol CH2(g)��H2(g)�ġ�H= kJ/mol��
CH2(g)��H2(g)�ġ�H= kJ/mol�� HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��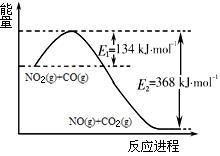
 2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���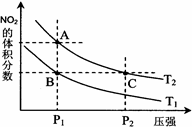
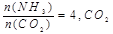 ��ת������ʱ��ı仯��ϵ��ͼ1��ʾ��
��ת������ʱ��ı仯��ϵ��ͼ1��ʾ�� ������ΪV����CO2��������ڡ�����С�ڡ����ڡ���
������ΪV����CO2��������ڡ�����С�ڡ����ڡ���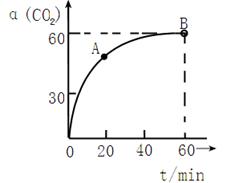
 CH3OH(g) ��H1
CH3OH(g) ��H1
 2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1
2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1
 ���ʱ�Ϊ��H = ��384 kJ��mol-1
���ʱ�Ϊ��H = ��384 kJ��mol-1