��Ŀ����
11��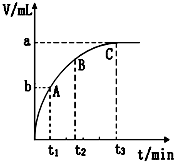 ij������ȤС���H2O2�ķֽ�������������ʵ��̽����
ij������ȤС���H2O2�ķֽ�������������ʵ��̽������1���±��Ǹ�С���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ���10mL H2O2��ȡ150mLO2�����ʱ�䣨�룩
| 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 | |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������� | 360 | 480 | 540 | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
�ڴ�����Ӱ��H2O2�ֽ����ʵ�����a��b����ѡһ����˵�������ضԸ÷�Ӧ���ʵ�Ӱ�죺�����������䣬���߷�Ӧ�¶ȣ�H2O2�ֽ����ʼӿ죮
��2��ijͬѧ��10mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ��
��д��H2O2�����Ļ�ѧ��Ӧ����ʽ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��A��B��C��������ʾ�ļ�ʱ��Ӧ������������C��
��3����������MnO2�����ữ��H2O2����Һ�У�MnO2�ܽ����Mn2+�����⣬�����л���O2��������д���÷�Ӧ�����ӷ���ʽMnO2+2H++H2O2=Mn2++O2��+2H2O��
���� ��1���ٹ���������Һ��ʵ������ȡ��������ҪҩƷ���ڳ����º��ѷֽ�õ���������ֽ��ٶ���Ũ�ȡ��¶ȡ����������ص�Ӱ�죻
�����ʵ�鷽����֤��ʱ��Ҫע��ʵ��Ŀ��Ʊ�������ȷ��ʵ������ȷ�ԣ�
��2���ٶ��������ǹ�������ֽ�Ĵ������ɴ�����ľ����ȼ����֪����ɴ˿�д������ʽ��
�ڸ���Ũ�ȶԷ�Ӧ���ʵ�Ӱ�������
���ữ��H2O2����MnO2��Ӧ����Mn2+��O2��
��� �⣺��1���ٸ��ݱ��и��������ݣ����������ȵ�����£���ͬŨ�ȵĹ���������Һ���Ǽ�������Ӧ�����������ȵ�����£���ͬŨ�ȵĹ���������Һ���ֽ⣬˵����������ķֽ��������¶��йأ����ǵõ���ͬ�����ʱ�䲻ͬ��Ũ��Խ��Ӧ���ٶ�Խ�죬˵����������ķֽ�������Ũ���йأ��Ƚ�ͬһŨ�ȵĹ���������Һ��30%ʱ�����������ȵ�ʱ����Ҫʱ����360s���д������ȵ������£���Ҫʱ����10s��˵����������ķֽ��������¶ȡ������йأ��ʴ�Ϊ���¶ȣ�������
�ڷ����������ݿ��Կ�����Ũ��Խ��Ӧ����Խ�죬�����ܼӿ��������ķֽ⣬�д����Ƿֽ����ʿ죬
�ʴ�Ϊ���¶�����ѧ��Ӧ���ʼӿ죻��Ӧ��Ũ������ѧ��Ӧ���ʼӿ죻ʹ�ú��ʵĴ�����ѧ��Ӧ���ʼӿ죻
��2����H2O2�ڶ������������·�����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
����������������ͬʱ���淴Ӧ�Ľ��У���ҺŨ�ȼ�С����ʱ��Ӧ������С��C��б��Ҳ��С���ʷ�Ӧ�����������ʴ�Ϊ��C��
���ữ��H2O2����MnO2��Ӧ����Mn2+��O2����Ӧ�ķ���ʽΪ��MnO2+2H++H2O2=Mn2++O2��+2H2O���ʴ�Ϊ��MnO2+2H++H2O2=Mn2++O2��+2H2O��
���� ����ͨ��ͼ�����ݣ���������������Է�Ӧ���ʵ�Ӱ�죬�Ѷ����У�Ҫע���������������ͬ��ֻ��һ�������ı�ʱ�����Ӱ�췴Ӧ���ʣ�

| A�� | ̼ | B�� | ���� | C�� | ���� | D�� | �Ҵ� |
| ʵ����� | ʵ��Ŀ�� | |
| A | ��±�������뵽��NaOH��Һ��һ��ʱ���ȡ�ϲ�Һ�壬����AgNO3Һ�������� | ֤��±�����к���±Ԫ�� |
| B | ���ϩȩ��CH2=CH-CHO���м�����������������Һ����KMnO4��H+����Һ���۲���ɫ��ȥ | ֤���ṹ�д���̼̼˫�� |
| C | �����������������ˮ�⣬������������ͭ����Һ���뵽ˮ������Һ�� | �������ˮ��IJ��������� |
| D | ���еμӴ��ᣬ��������������ͨ�뱥��̼��������Һ��ͨ�뱽����Ũ��Һ | ֤�����ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
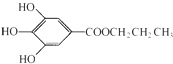 ���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������
���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������
 ij�¶�ʱ�����ݻ�Ϊ3L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ�����ݻ�Ϊ3L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ�����
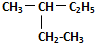 ��
��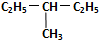 ��
��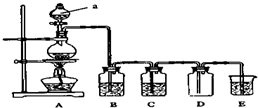 ��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ��
��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ��