��Ŀ����
3������ͭ���仯�������ճ��������������Ź㷺��Ӧ�ã���ش��������⣺��1������Ԫ�����ڱ��е�λ�õ������ڵڢ��壮
��2�������Fe��CO��x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��x�������ڷ��Ӿ��壨������ͣ���Fe��CO��x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x=5��Fe��CO��x��һ�������·�����Ӧ��
Fe��CO��x��s��?Fe��s��+xCO��g������֪��Ӧ������ֻ������λ�����ɴ��жϸ÷�Ӧ���γɵĻ�ѧ������Ϊ��������
��3��д��CO��һ�ֳ����ȵ�������ӵĽṹʽN��N��������ȽϷе�ϸߵ�ΪCO���ѧʽ����CN-��̼ԭ���ӻ��������Ϊsp��S��N��O��Ԫ�صĵ�һ����������ΪN����Ԫ�ط��ű�ʾ����
��4��ijMԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ��ʾ���ڵ����ͭԭ�ӣ���
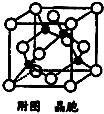
�ٸþ���Ļ�ѧʽΪCuCl��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ��������ڹ��ۣ�����ӡ��������ۡ��������
���� ��1��Feλ�ڵ������ڵ�VIII�壻
��2�����Ӿ����۷е�ϵͣ�Feԭ�Ӽ۵�������8��ÿ��CO�����ṩһ�����Ӷԣ��ݴ˼���nֵ��ֻ����λ�����ѣ����γɵĻ�ѧ���ǽ�������
��3��ԭ�Ӹ�����ȡ��۵�������ȵ����ǵȵ����壻���Է��ӵ��۷е�ϸߣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ���ʽ��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�ͬһ����Ԫ�أ���һ����������ԭ�������������С��
��4�������þ�̯�����㣻
�ڵ縺�Բ�С��1.7�Ļ�����Ϊ���ۻ����
��� �⣺��1��Feλ�ڵ������ڵ�VIII�壬�ʴ�Ϊ���������ڵڢ��壻
��2��Fe��CO��x������۷е�ϵͣ��������ڷ��Ӿ��壻Feԭ�Ӽ۵�������8��ÿ��CO�����ṩһ�����Ӷԣ�����8+2n=18��n=5��ֻ����λ�����ѣ����γɵĻ�ѧ���ǽ�������
�ʴ�Ϊ�����Ӿ��壻5����������
��3��ԭ�Ӹ�����ȡ��۵�������ȵ����ǵȵ����壬������CO�ǵȵ�����ķ��ӽṹʽΪN��N�����Է��ӵ��۷е�ϸߣ�CO�Ǽ��Է��ӣ������ǷǼ��Է��ӣ�����CO�۷е�ϸߣ�������Cԭ�Ӽ۲���ӶԸ�����2������Cԭ�Ӳ���sp�ӻ���ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�ͬһ����Ԫ�أ���һ����������ԭ�������������С�����Ե�һ������������NԪ�أ�
�ʴ�Ϊ��N��N��CO��sp��N��
��4��ijMԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����MΪClԪ�أ�
�ٸþ�����ͭԭ�Ӹ���=4��Clԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������仯ѧʽΪCuCl���ʴ�Ϊ��CuCl��
��Cu��ClԪ�صĵ縺�Բ�Ϊ3.0-1.9=1.1��1.7������Ϊ���ۻ�����ʴ�Ϊ�����ۣ�
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ���ӻ���ʽ�жϡ���ѧ����������֪ʶ�㣬���þ�̯�����۲���ӶԻ������۵�֪ʶ�㼴�ɽ���ѵ��Ǿ������㣬��Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ѧ��Ӧ�У��з��ȷ�ӦҲ�����ȷ�Ӧ | |
| B�� | ú��ʯ�͡���Ȼ���ǵ�����������Ҫ�����ֻ�ʯȼ�� | |
| C�� | Ba��OH��2•8H2O������NH4Cl����ķ�Ӧ�����ȷ�Ӧ | |
| D�� | ��ѧ��Ӧ�������仯�Ĵ�С�뷴Ӧ������������� |
 ij������ȤС���H2O2�ķֽ�������������ʵ��̽����
ij������ȤС���H2O2�ķֽ�������������ʵ��̽������1���±��Ǹ�С���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ���10mL H2O2��ȡ150mLO2�����ʱ�䣨�룩
| 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 | |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������� | 360 | 480 | 540 | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
�ڴ�����Ӱ��H2O2�ֽ����ʵ�����a��b����ѡһ����˵�������ضԸ÷�Ӧ���ʵ�Ӱ�죺�����������䣬���߷�Ӧ�¶ȣ�H2O2�ֽ����ʼӿ죮
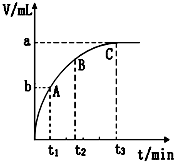
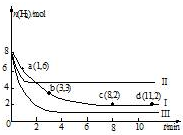
��2��ijͬѧ��10mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ��
��д��H2O2�����Ļ�ѧ��Ӧ����ʽ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��A��B��C��������ʾ�ļ�ʱ��Ӧ������������C��
��3����������MnO2�����ữ��H2O2����Һ�У�MnO2�ܽ����Mn2+�����⣬�����л���O2��������д���÷�Ӧ�����ӷ���ʽMnO2+2H++H2O2=Mn2++O2��+2H2O��
| A�� | ���ά�����ò���Ϊ����� | |
| B�� | SiO2��������������Բ����κ��ᷴӦ | |
| C�� | ����Na2SiO3��Һ��CO2��Ӧ���Ʊ�H2SiO3 | |
| D�� | NaOH��Һ����ʢװ�ڴ���������ĥ���Լ�ƿ�� |
 ������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺
������Ԫ��X��Y��Z��W��Ԫ�����ڱ������λ����ͼ��ʾ������Y������������������������ȣ���Ҫ��ش��������⣺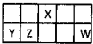
��1��д��X��ԭ�ӽṹʾ��ͼ
 ��
����2��Y��Z��W�ļ����Ӱ뾶�ɴ�С˳��ΪS2-��Cl-��Al3+�������ӷ��ű�ʾ����
��3����Y��ij���γ�������ˮ�����侻ˮԭ����Al3+3H2O?Al��OH��3�����壩+3H+�������ӷ���ʽ��ʾ����
��������ѧ��ѧԭ��������������⣺
��4����֪����C��s��+O2��g���TCO2��g������H=a kJ•mol-1��
��CO2��g��+C��s���T2CO��g������H=b kJ•mol-1��
��Si��s��+O2��g���TSiO2��s������H=c kJ•mol-1��
��ҵ�������ֹ���Ȼ�ѧ����ʽΪ2C��s��+SiO2��s��=2CO��g��+Si��s����H=��a+b-c��kJ•mol-1��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
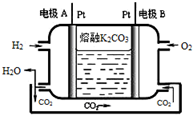
��6��������K2CO3Ϊ����ʵ�һ����������ȼ�ϵ�ع���ԭ����ͼ��ʾ��д���缫A�ĵ缫��ӦʽH2-2e-+CO32-�TCO2+H2O��
 һ���¶��£����ݻ�Ϊ2L�ĺ����ܱ������г���6molCO2��8molH2��������Ӧ��
һ���¶��£����ݻ�Ϊ2L�ĺ����ܱ������г���6molCO2��8molH2��������Ӧ��CO2��g��+3H2?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�����n��H2����ʱ��仯������I��ʾ������˵����ȷ���ǣ�������
| A�� | �÷�Ӧ��0��8min��CO2��ƽ����Ӧ������0.375mol•L-1•min-1 | |
| B�� | ����ʼʱ�����������г���3molCO2��4molH2����ƽ��ʱH2��������������20% | |
| C�� | ����ʼ�������������г���4molCO2��2molH2��2molCH3OH��1molH2O��g��������Ƿ�Ӧ������Ӧ������� | |
| D�� | �ı������õ�����II��III��������II��III�ı�����ֱ��������¶ȡ��������� |
| A�� | 6.72L CO | B�� | 6.6g CO2 | C�� | 8 g SO2 | D�� | 9.6g H2SO4 |
 ��������̼ԭ���ϵ�����Խϴ������ȩ��±�����ȷ�����Ӧ��
��������̼ԭ���ϵ�����Խϴ������ȩ��±�����ȷ�����Ӧ��
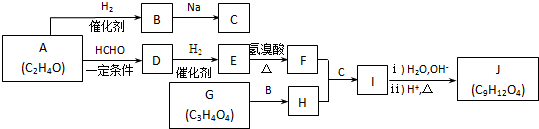
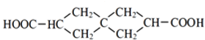 ��J��ͬ���칹��X���ڷ����廯���1mol X�ֱ�������Na��NaOH��Ӧʱ�����ʵ���֮����1��4��1��1���ұ�����ֻ��һ��һ�ȴ����������������X��2�֣�д������һ�ֵĽṹ��ʽ
��J��ͬ���칹��X���ڷ����廯���1mol X�ֱ�������Na��NaOH��Ӧʱ�����ʵ���֮����1��4��1��1���ұ�����ֻ��һ��һ�ȴ����������������X��2�֣�д������һ�ֵĽṹ��ʽ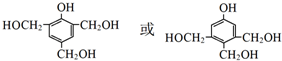 ��
�� ��ʵ������ȡ����������A�ķ�����2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�����
��ʵ������ȡ����������A�ķ�����2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�����