��Ŀ����
10��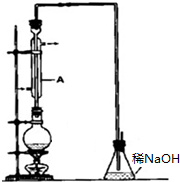 ��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ��ʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������������£�
��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ��ʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������������£��ټ��װ�õ������ԣ�����Բ����ƿ�м���95%�Ҵ���80%���ᣬȻ�������ϸ���廯�Ʒ�ĩ�ͼ������Ƭ����С�ļ��ȣ�ʹ���ַ�Ӧ����ش��������⣮
��1��װ��A������������������
��2����Ӧʱ���¶ȹ��ߣ�����SO2���ɣ�ͬʱ�۲쵽����һ�ֺ���ɫ���������������ķ���ʽ��Br2��
��3����Ӧ�����õ��Ĵֲ�Ʒ���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�a ����д����ȷѡ�����ĸ����a������������Һ b���Ҵ� c�����Ȼ�̼
��ʵ��������������Ҫ���������Ƿ�Һ©���������������ƣ���
��4��Ҫ��һ���Ƶô����������飬�ɼ���������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2��Ȼ����е�ʵ������ǣ�b����д��ȷѡ�����ĸ����a����Һ b������ c����ȡ
��5��Ϊ�˼����������к�����Ԫ�أ�����ֱ�����������еμ���������Һ�����飬��ԭ���������鲻������������Һ��Ӧ���ɳ�����ͨ�����õķ�����ȡ���������飬Ȼ��ܢ٢ۢڣ���ʵ��IJ���˳��ѡ��������ţ���
�ټ��� �ڼ���AgNO3��Һ �ۼ���ϡHNO3�ữ �ܼ���NaOH��Һ��
���� ��1�����������ܵ�����������������
��2��Ũ������������ԣ����Խ���ԭ�Ե�����������Ϊ�嵥�ʣ�
��3���嵥���ܽ����л�������ʾ�ػ�ɫ���嵥�ʿ��Ժ��������Ʒ�����Ӧ��ʵ�ֻ�������Һ��ķ�����÷�Һ©������Һ��
��4�������ܽ�����ʵķ����������
��5�������������к�����Ԫ��һ��Ҫ��֮ת��Ϊ�����ӣ����������ӿ��Ժ������ӷ�Ӧ���ɵ���ɫ����������ij��������飮
��� �⣺��1��ʵ��ʱ�����������ܵ��������������������Ի��Ҫ��ȡ���л��
�ʴ�Ϊ������������
��2��Ũ������������ԣ����Խ���ԭ�Ե�����������Ϊ�嵥�ʣ��õ��ĺ���ɫ����Ϊ��������Ũ������������ԭΪ��������
�ʴ�Ϊ��Br2��
��3���嵥���ܽ����л�������ʾ�ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е������嵥�ʣ�������������������֮������Ӧ��ȥ����Ӧ�����Һ�������黥�����ܣ�����Բ��÷�Һ�����÷�Һ©������Һ��
�ʴ�Ϊ��a����Һ©����
��4��������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2��������ķе�ϵͣ����Բ������������룬
�ʴ�Ϊ��b��
��5��Ϊ�˼����������к�����Ԫ�أ�����ֱ�����������еμ���������Һ�����飬����Ϊ������Ϊ�ǵ���ʣ������鲻������������Һ��Ӧ���ɳ����������������к�����Ԫ��һ��Ҫ��֮ת��Ϊ�����ӣ����Բ���±������ˮ�ⷽ�����������������Ƽ��ɣ��������ӿ��Ժ������ӷ�Ӧ���ɵ���ɫ����������ij����廯�������飬���Լ��������ữ����������
�ʴ�Ϊ�������鲻������������Һ��Ӧ���ɳ������ܢ٢ۢڣ�
���� ���⿼�������������ȡ��������Ŀ�Ѷ��еȣ���ȷ�Ʊ�ԭ������ѧʵ�������������Ϊ���ؼ�������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ�����������ѧʵ��������

| A�� | D2+��B2+��A2+��E2+ | B�� | B2+��A2+��D2+��E2+ | C�� | D2+��E2+��A2+��B2+ | D�� | E2+��B2+��A2+��D2+ |
| A�� | ���������������Ȼ�茶����� | B�� | ���ȷ�Ӧ | ||
| C�� | þ������ķ�Ӧ | D�� | ���������� |
�ټ���
�ڼ����������ữ��AgNO3��Һ���۲��Ƿ����dz��ɫ����
�ۼ���NaOH��Һ
��ȷ�IJ���˳���ǣ�������
| A�� | �٢ڢ� | B�� | �ڢ٢� | C�� | �ۢ٢� | D�� | �ۢڢ� |
| A�� | 96% | B�� | 48% | C�� | 9.6% | D�� | 56% |
| A�� | ${\;}_{17}^{37}$Cl2��Ħ��������74 | |
| B�� | ${\;}_{17}^{37}$Cl��${\;}_{17}^{35}$Cl��Ϊͬλ�أ�${\;}_{17}^{35}$Cl2��Cl2��Ϊͬ���칹�� | |
| C�� | ͨ������£���������������������Ҳ���л�ԭ�� | |
| D�� | ��ʹʪ��ĵ���KI��ֽ����ɫ������һ����Cl2 |
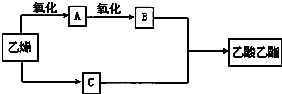

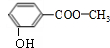 ��EHCHO��
��EHCHO��