��Ŀ����
2��ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�CH3CH=CH2+CO+H2$\stackrel{һ������}{��}$CH3CH2CH2CHO$��_{Ni����}^{H_{2}}$CH3CH2CH2CH2OH
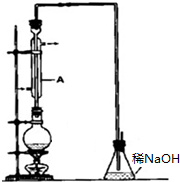
CO���Ʊ�ԭ����HCOOH$��_{��}^{Ũ����}$CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã���ͼ��

����д���пհף�
��1��ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2-����������ѡ����ʵ��Լ��Ʊ���������ϩ��д����ѧ����ʽ��Zn+2HCl=ZnCl2+H2������CH3��2CHOH$��_{��}^{����}$CH2=CHCH3��+H2O��
��2����������װ���Ʊ����﴿����CO��װ����a��b�����÷ֱ��Ǻ�ѹ����������
C��d�г�װ���Լ��ֱ���NaOH��Һ��ŨH2SO4����������װ���Ʊ�H2�����巢��װ���б���IJ������������Ƿ�Һ©����������ƿ�������߿��ڻ����ռ�����H2��װ��ͼ��
��3���Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳���Ǣܢݢ٢ڢۣ���ܢݢ٢ۢڣ�������ţ�
�ٱ���Na2SO3��Һ������KMnO4��Һ��ʯ��ˮ����ˮCuSO4��Ʒ����Һ
��4���ϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�����ΪӦ�ò��õ����˷�Ӧ������b��
a�����¡���ѹ������ b���ʵ����¶ȡ���ѹ������
c�����¡���ѹ������ d���ʵ����¶ȡ���ѹ��������
���� ��1���Ʊ�����ѡ��п����ϡ����Ʊ���ϩѡ��2-������Ũ���
��2�������װ���У�a�����ñ��ַ�Һ©������ƿ�ڵ���ѹ��ȣ��Ա�֤��Һ©���ڵ�Һ����˳��������ƿ�У�b��Ҫ����ȫƿ�����ã��Է�ֹ������cΪ��ȥCO�е��������壬ѡ��NaOH��Һ��dΪ��ȥCO�е�H2O���Լ�ѡ��Ũ����������װ���Ʊ�H2������Ҫ�ƾ��ƣ�
��3�������ϩ������SO2��CO2��ˮ������ɵĻ��������ɷ�ʱ��Ӧ����ѡ����ˮCuSO4����ˮ������Ȼ���â�Ʒ����Һ����SO2�����âٱ���Na2SO3��Һ��ȥSO2��Ȼ���â�ʯ��ˮ����CO2���â�����KMnO4��Һ�����ϩ��
��4������ϳ�����ȩ�ķ�ӦΪ���������С�ķ��ȷ�Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�
��� �⣺��1���������û��ý���п���������������ͨ���û���Ӧ�Ʊ������������������Ũ������п��Ӧ���ܲ�������������ʽΪZn+2HCl=ZnCl2+H2����2-����ͨ����ȥ��Ӧ�������ϩ������ʽΪ ��CH3��2CHOH$��_{��}^{����}$CH2=CHCH3��+H2O��
�ʴ�Ϊ��Zn+2HCl=ZnCl2+H2���� ��CH3��2CHOH$��_{��}^{����}$CH2=CHCH3��+H2O��
��2��������Ũ�����������ͨ��������ˮ������CO�����ڼ����ӷ���������CO�б�Ȼ����м��ᣬ�������ռ�֮ǰ��Ҫ��ȥ���ᣬ��������NaOH��Һ���ռ��ᣮ����Ϊ����������ˮ�����Ա����ֹҺ�嵹������b�������Ƿ�ֹ���������ͨ��Ũ�������CO��Ϊ��ʹ������������˳���Ĵӷ���װ�����ų����ͱ��豣��ѹǿһ�£����a�������DZ��ֺ�ѹ����������װ���Ʊ��������Ͳ�����Ҫ���ȣ����Դ�ʱ����װ���еIJ������������Ƿ�Һ©����������ƿ�������ܶ�С�ڿ����ģ����Ҫ�ռ��������������ֻ���������ſ�����������������ˮ���ռ���
�ʴ�Ϊ����ѹ���������� NaOH��Һ��ŨH2SO4����Һ©����������ƿ��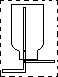 ��
��
��3�������ϩ����������KMnO4��Һ������SO2����������KMnO4��Һ��ɫ��Ʒ����Һ��ʯ��ˮ������CO2����ʯ��ˮ������ˮ����������ˮCuSO4�������ڼ���������������迼���Լ���ѡ���˳��ֻҪͨ����Һ���ͻ����ˮ����������ȼ���ˮ������Ȼ�����SO2���ڼ���֮���ȥSO2����SO2�����ñ���Na2SO3��Һ��������CO2�ͱ�ϩ�����˳��Ϊ�ܢݢ٢ڢۣ���ܢݢ٢ۢڣ���
�ʴ�Ϊ���ܢݢ٢ڢۣ���ܢݢ٢ۢڣ���
��4�����ڷ�Ӧ��һ�������С�Ŀ��淴Ӧ�����Բ��ø�ѹ������������Ӧ���ʺ����ԭ������ת���ʣ�����Ӧ�Ƿ��ȷ�Ӧ����Ȼ�������������ԭ������ת���ʣ�������������Ӧ���ʣ����Ҫ�����ʵ����¶ȣ������������ԭ������ת���ʣ�������������Ӧ���ʣ����̵���ƽ������Ҫ��ʱ�䣬����ȷ��ѡ����b��
�ʴ�Ϊ��b��
���� ���⿼���л���ϳɷ�������ƣ���Ŀ�ѶȽϴ��ۺ��Խ�ǿ������ʱע��������ʵķ��롢�ᴿ�������������ʵ����ʵ���ͬ�ǽ�����Ĺؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �����Ǻ��� | B�� | �Ҵ������� | C�� | ���Ǻ���ѿ�� | D�� | ����ͼ������ |
| A�� | Fe2++SO42-+Ba2++2OH-�TBaSO4��+Fe��OH��2�� | |
| B�� | NH4++Fe3++2SO42-+2Ba2++4OH-�T2BaSO4��+Fe��OH��3��+NH3•H2O | |
| C�� | 2Fe3++3SO42-+3Ba2++6OH-�T3BaSO4��+2Fe��OH��3�� | |
| D�� | 3NH4++Fe3++3SO42-+3Ba2++6OH-�T3BaSO4��+Fe��OH��3��+3NH3•H2O |
��1����ͬѧ��Ϊ�ǣ�Br2����ˮ��ɻ�ɫ��Һ������Ϊ�ǣ�Fe2+��������Fe3+ʹ��Һ��ɻ�ɫ��
��2�����ṩ�Լ������Ը��������Һ������������Һ�����Ȼ�̼�����軯����Һ
�������ַ���������֤��д��ѡ�õ��Լ���ż�ʵ���й۲쵽������
| ѡ���Լ� | ʵ������ | |
| ��һ�ַ��� | ���Ȼ�̼ | |
| �ڶ��ַ��� | ���軯����Һ |
��4����ϡ�廯������Һ��ͨ��������������ӷ�Ӧ����ʽ��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl-��
�����ȡ��Ʒ1.500g��
�������Ʒ�ܽ����ȫת�Ƶ�250mL����ƿ�У����ݣ����ҡ�ȣ�
�������ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У�����10mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㣮�����������������ظ�2�Σ�
��1�����ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�������Ӱ�죨�ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�B��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ���������һ��NaOH��Һ���£���Һ����ɫ���ۺ죨��dz�죩���Ұ�����ڲ���ɫ��
��2���ζ�������±���ʾ��
| �ζ� ���� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
 ��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ��ʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������������£�
��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ��ʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������������£�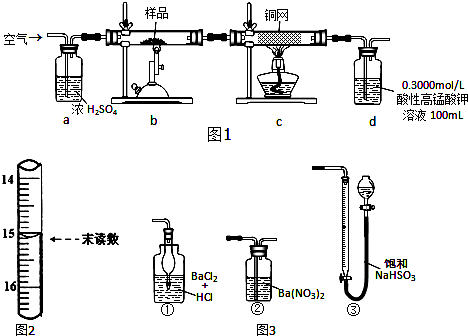


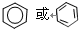 ��
�� ��Na��F������ȼ�յIJ�����Na2O2��
��Na��F������ȼ�յIJ�����Na2O2��