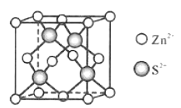
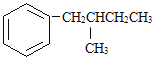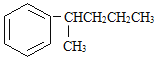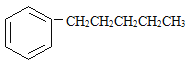��Ŀ����
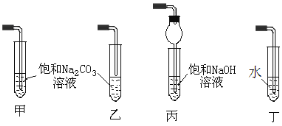
����Ŀ��(1)��A������ B������ C���Ҵ� D�������ʵ������л����У�ѡ����ʵ����ʣ����������ں����ϡ�
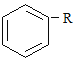
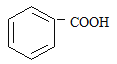
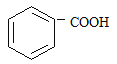
����˿����Ҫ�ɷ���__________��
���ҹ���������������������Ҫ�ɷ���____________��
���Ƽ���ָ��ʻԱ������������____________�������ꡣ
���������ˮƿ�ڵ�ˮ��[��Ҫ�ɷ�CaCO3��Mg(OH)2]����____________��
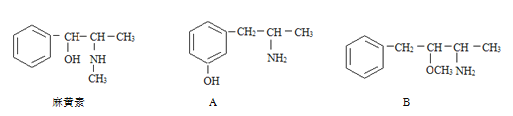
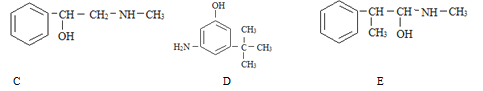
(2)A��B��C��D��E��Ϊ��ѧ�����л����ת����ϵ����ͼ���ش��������⣺
![]()
��E���ʵĽṹ��ʽΪ____________��AB�Ļ�ѧ����ʽΪ____________��
��ʵ��������A��C��ȡD����Ӧ�Ļ�ѧ����ʽΪ_____���ռ�װ�ÿ�ѡ����ͼ______װ��(����)��
���𰸡�D A C B ![]() 2C2H5OH+O2
2C2H5OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ��
CH3COOCH2CH3+H2O ��
��������
(1) ����˿����Ҫ�ɷ��ǵ����ʣ����ҹ���������������������Ҫ�ɷ��Ǽ��飻���Ƽ���ָ��ʻԱ�����������оƾ����Ҵ��ĺ������ꣻ���������ˮƿ�ڵ�ˮ��[��Ҫ�ɷ�CaCO3��Mg(OH)2]�������ᣬ�ʴ�Ϊ��D��A��C��B��
(2)��ϩ��ˮ�����ӳɷ�Ӧ�õ��Ҵ����Ҵ������õ���ȩ����ȩ�������õ����ᣬ�Ҵ������� ����������Ӧ�õ�������������ϩ�����Ӿ۷�Ӧ�õ�����ϩ��
��EΪ����ϩ���ṹ��ʽΪ��![]() ��AB���Ҵ�����������ȩ�Ĺ��̣���ӦΪ��2C2H5OH+O2
��AB���Ҵ�����������ȩ�Ĺ��̣���ӦΪ��2C2H5OH+O2![]() 2CH3CHO+2H2O���ʴ�Ϊ��
2CH3CHO+2H2O���ʴ�Ϊ��![]() ��2C2H5OH+O2
��2C2H5OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��ʵ��������A��C��ȡD�����Ҵ������ᷢ��������Ӧ�õ�������������ӦΪ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���ռ�װ��Ӧѡ���ң�����̼������Һ�����ܽ��Ҵ����к����ᣬ���������������ܽ�ȱ�����������������Һ���Ͽ��Է���ֹ������ѡ�ң���װ�õ���������Һ�л����������ʲ�ѡ����װ���������ƻᵼ����������ˮ�⣬�ʲ�ѡ����װ��ˮ������Ч��ȥ�Ҵ������ᣬ�ʲ�ѡ���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���ռ�װ��Ӧѡ���ң�����̼������Һ�����ܽ��Ҵ����к����ᣬ���������������ܽ�ȱ�����������������Һ���Ͽ��Է���ֹ������ѡ�ң���װ�õ���������Һ�л����������ʲ�ѡ����װ���������ƻᵼ����������ˮ�⣬�ʲ�ѡ����װ��ˮ������Ч��ȥ�Ҵ������ᣬ�ʲ�ѡ���ʴ�Ϊ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���ң�
CH3COOCH2CH3+H2O���ң�

����Ŀ���ڼס��ҡ���������ͬ�ܱ������а���ͬ��ʽͶ�ϣ�һ�������·�����Ӧ(��ʼ�¶Ⱥ���ʼ�����ͬ):N2(g)+3H2(g)![]() 2NH3(g) ��H<0������������±���ʾ:
2NH3(g) ��H<0������������±���ʾ:
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | lmolN2��3molH2 | 2molNH3 | 2molNH3 |
ƽ��ʱ������� | V�� | V�� | V�� |
��Ӧ��ƽ�ⳣ��K | K�� | K�� | K�� |
ƽ��ʱNH3��Ũ��/mol/L | c�� | c�� | c�� |
ƽ��ʱNH3�ķ�Ӧ����/mol/(L��min) | v�� | v�� | v�� |
����˵����ȷ����
A. V��>V�� B. K��>K�� C. c��>c�� D. V��=V��