��Ŀ����
����Ŀ��һ�Ȼ���![]() ��һ����Ҫ��±�ػ�����ڻ����������й㷺Ӧ�á��й�һ�Ȼ���IJ�����Ϣ���£�
��һ����Ҫ��±�ػ�����ڻ����������й㷺Ӧ�á��й�һ�Ȼ���IJ�����Ϣ���£�

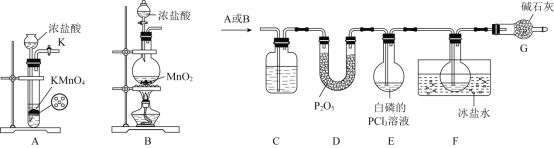
ijС�������ʵ���Ʊ�һ�Ȼ��Ⲣ�ⶨ�䴿�ȣ�װ����ͼ��ʾ��

����ͼ��ʾװ�ý���ʵ�飬��Dװ���в�������ɫҺ�塢������ʧʱֹͣ��Ӧ��
��ش��������⣺
(1)ʢװ�ⵥ�ʵ�����������_____________��Eװ��������_____________��
(2)![]() װ�����Լ�������______________________________��
װ�����Լ�������______________________________��
(3)д��A�з�Ӧ�����ӷ���ʽ______________________��
(4)������Ӧ��Dװ�õ�������������ˮԡ�У���Ŀ����__________________��
(5)�ֲ�Ʒ�л��еⵥ�ʣ��ᴿ��Ʒ��ѡ������װ���е�_____________![]() ����ĸ
����ĸ![]() ��
��

(6)�ⶨ��Ʒ���ȡ�ȡ![]() �ò�Ʒ����ƿ�����������KI��Һ����ַ�Ӧ����
�ò�Ʒ����ƿ�����������KI��Һ����ַ�Ӧ����![]() ��Һ�ζ�
��Һ�ζ�![]() �йط�Ӧ��
�йط�Ӧ��![]() ��
��![]() ������ƽ��ʵ�����������£�
������ƽ��ʵ�����������£�
![]()
�ò�Ʒ����Ϊ___________![]() �ú�a��c�Ĵ���ʽ��ʾ
�ú�a��c�Ĵ���ʽ��ʾ![]() ������Ʒ�л�������
������Ʒ�л�������![]() ����ý��_______________
����ý��_______________![]() ����ƫ������ƫ����������Ӱ����
����ƫ������ƫ����������Ӱ����![]() ��
��
���𰸡�Բ����ƿ ����Fװ�ûӷ�������ˮ��������ֹICl��ˮ��Ӧ ����ʳ��ˮ ![]()
![]()
![]() c ��ȴ��Ʒ�����ٲ�Ʒ�ӷ�
c ��ȴ��Ʒ�����ٲ�Ʒ�ӷ� ![]() ƫ��
ƫ��
��������
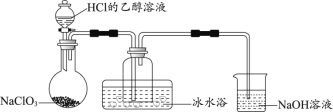
����A��ȡ�����������к���HCl��H2O�����ʣ���Ҫ��ȥ�����ñ���ʳ��ˮ����ȥHCl������Ũ�����ȥH2O����D�з�����Ӧ�õ�ICl�����ڲ�����ˮ��Ӧ���ڽ���β������ʱ��Ҫ��ֹˮ����������D�������D��β������װ��֮�������һ������װ�á�
(1)����װ��ͼ������֪װ��D������ΪԲ����ƿ��װ��E������������Fװ�ûӷ�������ˮ��������ֹICl��ˮ��Ӧ���ʴ�Ϊ��Բ����ƿ������Fװ�ûӷ�������ˮ��������ֹICl��ˮ��Ӧ��
(2)Bװ�õ������dz�ȥ�����е��Ȼ������壬��˿���ѡ�ñ���ʳ��ˮ���ʴ�Ϊ������ʳ��ˮ��
(3)Aװ��������ȡ��������Ӧ�����ӷ���ʽΪ��MnO2+2Cl-+4H+![]() Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+2Cl-+4H+
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+2Cl-+4H+![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
(4)����һ�Ȼ�������������Լ��Ʊ�һ�Ȼ���ķ�Ӧ���ȷ�����֪������Ӧ��Dװ�õ�������������ˮԡ�У���Ŀ������ȴ��Ʒ�����ٲ�Ʒ�ӷ����ʴ�Ϊ����ȴ��Ʒ�����ٲ�Ʒ�ӷ���
(5)����һ�Ȼ���͵ⵥ�ʵ��������ʷ�����֪���ߵķ�����Բ�������ķ������ʴ�Ϊ��c��
(6)���ݷ�Ӧ��ԭ��������֪��Ӧת����ϵʽΪICl~I2~2Na2S2O3���ɱ�������֪�ڶ��βⶨ���ϴ���ȥ���ɵ�һ�κ͵�����ʵ������ɵ�һ�Ȼ�������ʵ���Ϊ![]() ��һ�Ȼ���Ĵ���Ϊ
��һ�Ȼ���Ĵ���Ϊ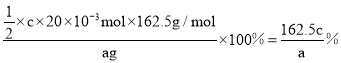 ������Ʒ�л�������I2����ICl��I2��Na2S2O3�Ĺ�ϵ��֪�����������������£��� I2Խ�࣬�����ĵ�Na2S2O3����Խ�٣���ý����ƫ�ͣ��ʴ�Ϊ��
������Ʒ�л�������I2����ICl��I2��Na2S2O3�Ĺ�ϵ��֪�����������������£��� I2Խ�࣬�����ĵ�Na2S2O3����Խ�٣���ý����ƫ�ͣ��ʴ�Ϊ��![]() ��ƫ�͡�
��ƫ�͡�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���������������������������������ӦΪ��2NO2(g)+O3(g)![]() N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ���ǣ� ��
N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ���ǣ� ��
A | B | C | D |
|
|
|
|
�����¶ȣ� | 0��3s�ڣ���Ӧ����Ϊ�� | t1ʱ����������� | ��ƽ��ʱ�����ı�x����xΪc(O2) |
����Ŀ��ClO2��Ϊһ�ֹ����͵�����������������ȡ��Cl2��Ϊ����ˮ������������֪ClO2��һ��������ˮ���������л��ܼ������壬11��ʱҺ���ɺ���ɫҺ�塣
��1��ij�о�С������ͼװ���Ʊ�����ClO2���г�װ������ȥ����
�ٱ�ˮԡ��������____________��
��NaOH��Һ����Ҫ����Ϊ���շ�Ӧ������Cl2��������Һ��������ȡƯ��Һ�������շ�Ӧ���������뻹ԭ��֮��Ϊ___________________��
����NaClO3��HClΪԭ���Ʊ�ClO2�Ļ�ѧ����ʽΪ_________________________��
��2����ClO2ˮ��Һ�μӵ�KI��Һ�У���Һ���ػƣ��������м�������CCl4�������ã��۲쵽________��֤��ClO2���������ԡ�
��3��ClO2��ɱ�����������л����Cl-,�京��һ�������0.3-0.5 mg��L1��ij�о�С��������ʵ�鷽���ⶨ���ڲ���ˮ������ˮ����Cl-�ĺ�������ȡ10.00 mL������ˮ����ƿ�У���K2CrO4Ϊָʾ������0.0001mol��L��1��AgNO3����Һ�ζ����յ㡣�ظ������������Σ�����������±���ʾ��
ʵ����� | 1 | 2 | 3 | 4 |
����AgNO3��Һ�����/mL | 10.24 | 10.02 | 9.98 | 10.00 |
���ڵζ�����װ��AgNO3����Һ��ǰһ����Ӧ���еIJ���_____________��
�ڲ������ˮ��Cl-�ĺ���Ϊ______ mg��L1��
�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ⶨ���_______������ƫ��������ƫ����������Ӱ��������