��Ŀ����
����Ŀ��ѧϰС��̽��AgNO3��Ag2O(�غ�ɫ���壬������ˮ)����ˮƯ���Ե�Ӱ�졣
ʵ���¼���£�
| ʵ�� | ���������� |
�� | ����1mL����ˮ���ٵμ�1��Ʒ����Һ��Ʒ����Һ�Ͽ���ɫ | |
�� | ��������Ag2O���壬������ɫ����a���ټ���1mL����ˮ�� 1��Ʒ����Һ��Ʒ����Һ��ɫ��i�� | |
�� | ����1mL��ŨAgNO3��Һ��������ɫ����b���ٵμ�1��Ʒ����Һ��Ʒ����Һ��ɫ��i�� |
(1)�����ӷ���ʽ��ʾi��Ʒ����Һ��ɫ��ԭ��______��
(2)�����飬ii�еİ�ɫ����a��AgCl������AgCl�Ļ�ѧ����ʽ��_______��
(3)����iii�ĶԱ�ʵ�飬Ŀ�����ų�iii��______��ɵ�Ӱ�졣
(4)�о���ɫ����b�ijɷ֡�����ʵ��iv����ʵ��iii�ķ����ٴεõ���ɫ����b�����ˡ�ϴ�ӣ������Թ��У���
ʵ��iv:
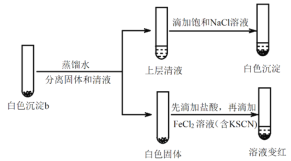
����ƶԱ�ʵ��֤ʵ��ɫ����b��ֻ����AgCl��ʵ�鷽����������_______��
��FeCl2��Һ��������_______���ɴ��жϣ���ɫ����b���ܺ���AgClO��
(5)��һ���о���ɫ����b��ʵ��iiiƷ����Һ��ɫ����ԭ����ʵ��v��
ʵ��v:

�ٽ�����ӷ���ʽ���ͼ��뱥��NaCl��Һ��Ŀ�ģ�_______��
���Ʋ�Ʒ����Һ��ɫ�����ʣ�ʵ��iii��ʵ��v______����족�� ����������
���𰸡�![]()
![]() ϡ�Ͷ���Һ��ɫ �ô�����AgCl���������ɫ����b���ȵμ����ᣬ�ٵμ�FeCl2��Һ����KSCN������Һ����졣 �����ɫ����b���Ƿ������������� AgClO(s) + Cl-(aq)
ϡ�Ͷ���Һ��ɫ �ô�����AgCl���������ɫ����b���ȵμ����ᣬ�ٵμ�FeCl2��Һ����KSCN������Һ����졣 �����ɫ����b���Ƿ������������� AgClO(s) + Cl-(aq) ![]() AgCl(s) + ClO-(aq)��ʹ��ɫ����b�е�ClO-�����ϲ���Һ���ڼ�� ��
AgCl(s) + ClO-(aq)��ʹ��ɫ����b�е�ClO-�����ϲ���Һ���ڼ�� ��
��������
��ˮ����Ư���ԣ�����Ϊ��ˮ�к���HClO����HClO��Դ��Cl2��ˮ�����Ŀ��淴Ӧ�����AgNO3��Ag2O����ˮƯ�����������Ӱ�죬���ܵ�ԭ��Ӱ����Cl2��ˮ�ķ�Ӧ����һ��ʵ������֤AgNO3��Ag2O����ˮƯ���Ե�Ӱ�죬������֤Ag2O��Ӱ��ʱ���������������ˮ�Ĺ��壬������Һ�������������Ӱ�죬����֤AgNO3��Ӱ��ʱ�����������������Һ����Һ����ᷢ���仯�����ڿ��Ʊ����Ŀ��ǣ�ʵ�颡��ʵ�颢�ֱ��ֵμ���1mLˮ���ų���Һ����仯��ʵ������Ӱ�졣ʵ�颢��ʵ�颣�������˰�ɫ����������ʵ�颢�еİ�ɫ������AgCl��Ϊ��̽��ʵ�颣�в����İ�ɫ�����ijɷ֣�����˵ڶ���ʵ�飬�����֪����ɫ����b���������Һ�л�����ܹ�ʹFe2+�����������ӣ������֤��ɫ����b����ɲ�������AgCl���Դ������Һ����ʹFe2+���������֡���ȷ����ɫ����b����ɺ�������˵�����ʵ�飬̽�����ɰ�ɫ����b��ʵ�颣��Ʒ����Һ��ɫ������ԭ��ʵ������У�����ɫ����Ͷ�뱥��NaCl��Һ�У���Ϊ�˽���ת��ΪAgCl��ʹClO-������Һ�б��ڼ�⣬ͬʱ����Ҳ��ʹ����Һ�е�ClO-Ũ�������ʵ�颣��ø������ʹƷ����Һ��ɫ���졣
(1)��ˮ����Ư���ԣ�����Ϊ���д����ᣬ��ص����ӷ���ʽΪ��![]() ��
��
(2)ͨ��������֪������AgCl�Ļ�ѧ����ʽΪ��![]() ��
��
(3)ͨ��������֪����1mL����ˮ��Ϊ���ų�ϡ�Ͷ���Һ��ɫ��Ӱ�죻
(4)��ͨ��������֪����֤��ɫ����b����ɲ�������AgCl���Դ������Һ����ʹFe2+���������֣�����ô�����AgCl�����ɫ����b���ȵμ�ϡ���ᣬ�ٵμ�FeCl2��Һ(��KSCN)����Һ����켴��֤����
��ͨ��������֪��FeCl2(��KSCN)��������ͨ���Ƿ��ɫ�������b���Ƿ������������ӣ�
(5)��ͨ��������֪������ɫ����Ͷ�뱥��NaCl��Һ�У���Ϊ�˽���ת��ΪAgCl��ʹClO-������Һ�б��ڼ�⣬��ص����ӷ���ʽΪ��![]() ��
��
��ͨ��������֪��ʵ�颥���ϲ���Һ�е�ClO-Ũ�������ʵ�颣�Ƚϴ����ʹƷ����Һ��ɫ�����ʵ�颣���졣