��Ŀ����
4����CH3COOOH������ɫ��Һ�壬������ˮ���ӷ����������ֽ⣬����ǿ�����Եĸ�Ч��������ʹ�ù�������������ʱͨ������ˮϡ��ҵƷ�������ᣬȻ�����������Կ�����������������
��1������220mL0.05mol/L�Ĺ���������Һ����ʹ�õ������У��ձ�����Ͳ����������250ml����ƿ����ͷ�ιܡ���2�����ƹ����У���������Ũ��ƫ�ߵIJ�����C
A������ƿ������ˮϴ�Ӻ�δ���������������ˮ
B��ת����Һʱ��������������Һ��������ƿ��
C������ʱ����������ƿ�̶��߽��ж���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ����伸��ˮ���̶���
��3������������ȷ����BC������ĸ��
A�����ù��˵ķ���������������Һ�л��е�NaCl����
B�����������װ��Ӧ����Σ�վ����ǩӦ����ͼ��ʾ
C����������Ӧע���ܱա����±�������ɫϸ��ƿ��
D������������һ���л��������������ȡ��ˮ�еĵⵥ��
��4�����������Сʱ�ڻ���ȫ�ֽ�����ᣨCH3COOH����һ�ֳ��������嵥�ʣ���������嵥�ʵ�ʵ�鷽�����ô����ǵ�ľ���Ӵ����壬�۲��Ƿ�ȼ��
��5������������������ԭ��CH3COONa�л�����SO42-��Ҫ�����SO42-��ѡ�������Լ����ռ����Ⱥ�˳����բڢݢܣ��Լ�����ѡ�꣬����ż��ɣ�
������ڴ��ᱵ��Һ���Ȼ�����Һ�ܴ����̼������Һ��̼��������Һ
��6����ȡ�������ᷴӦԭ��Ϊ��H2O2+CH3COOH$\stackrel{ŨH_{2}SO_{4}}{��}$CH3COOOH���������ᣩ+H2O���ֳ�ȡ5.0g������������ȡ�Ĺ�������������Һ�壩��ϡ����100mL���ã�ȡ����ϡ�ͺ������������5.0mL����0.01mol/L KMnO4��Һ�Գ�ȥ���е�H2O2���漴����10%KI��Һ10mL��ҡ�ȣ���ѧ��Ӧ����ʽΪCH3COOOH+2KI=CH3COOK+I2+KOH���ٽ�0.05mol/L Na2S2O3����Һ���뵽���������Һ�У���ѧ��Ӧ����ʽΪ��I2+2Na2S2O3=2NaI+Na2S4O6��������Na2S2O3����Һ�������Ϊ20mL��ͨ������ȷ��ԭ�����й����������������Ϊ15.2%��
���� ��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��3��A��NaCl�����ڹ������
B������������ǿ�����ԣ�
C������������Һ̬�������ֽ⣻
D����������������ˮ��
��4�����������ֽܷ�Ϊ���������������������ʹ�����ǵ�ľ����ȼ��������
��5����ȥCH3COONa�л��е�SO42-��Ӧ��������Ĵ��ᱵ��Ȼ���̼���Ƴ�ȥ�����ı����ӣ����Ӵ����ȥ������̼������ɣ�
��6�����ݣ�3���ķ���ʽ��I2+2S2O32-=2I-+S4O62-������ѧ����ʽ�����Եù�ϵʽ���£�CH3COOOH��I2��2Na2S2O3���ݴ˼���ϡ�ͺ�5mL��Ӧ��n��CH3COOOH������������ԭ�����й������������������
��� �⣺��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪�������������Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ��ʳ����ձ�����Ͳ���������⣬����Ҫ250ml����ƿ����ͷ�ιܣ��ʴ�Ϊ��250ml����ƿ����ͷ�ιܣ�
��2��A������ƿ������ˮϴ�Ӻ�δ���������������ˮ��������Һ��Ũ����Ӱ�죬��A����
B��ת����Һʱ��������������Һ��������ƿ�⣬��������ʵ���ʧ����Ũ��ƫ�ͣ���B����
C������ʱ����������ƿ�̶��߽��ж��ݣ�����Һ���ƫС��Ũ��ƫ�ߣ���C��ȷ��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶����������ģ����ز��伸��ˮ���̶��ߣ��������ˣ���Ũ��ƫ�ͣ���D����
��ѡC��
��3��A��NaCl�����ڹ������ᣬ�ʲ����ù��˵ķ�����ȥ���������е��Ȼ��ƣ���A����
B������������ǿ�����ԣ����������װ��Ӧ����Σ�վ����ǩӦ����ͼ��ʾ����B��ȷ��
C������������Һ̬�������ֽ⣬��Ӧ�ܹⱣ�棬��������Һ̬��Ӧ������ϸ��ƿ�У���C��ȷ��
D����������������ˮ���ʲ�����ȡ��ˮ�еĵ⣬��D����
��ѡBC��
��4�����������ֽܷ�Ϊ�������������������ʹ�����ǵ�ľ����ȼ���ʼ��������ķ���Ϊ���ô����ǵ�ľ���Ӵ����壬�۲��Ƿ�ȼ���ʴ�Ϊ���ô����ǵ�ľ���Ӵ����壬�۲��Ƿ�ȼ��
��5����ȥCH3COONa�л��е�SO42-��Ӧ���뱵�Σ����뱵���ӣ����Ӳ�Ҫ���������ʵĽǶ���������Ӧ��������Ĵ��ᱵ��Ȼ��ӹ�����̼���Ƴ�ȥ�����ı����ӣ����Ӵ����ȥ������̼������ɣ��ʴ�Ϊ���ڢݢܣ�
��6�����ݣ�3���ķ���ʽ��I2+2S2O32-=2I-+S4O62-������ѧ����ʽ�����Եù�ϵʽ���£�CH3COOOH��I2��2Na2S2O3���ɹ�ϵʽ��֪��n��CH3COOOH��=$\frac{1}{2}$n��Na2S2O3��=$\frac{1}{2}$��0.05mol/L��0.02L=5��10-4mol����ԭ��Ʒ��w��CH3COOOH��=$\frac{5��1{0}^{-4}mol��76g/mol}{5g��\frac{5mL}{100mL}}$��100%=15.2%��
�ʴ�Ϊ��15.2%��
���� ���⿼��������ԭ��Ӧ�ζ���ʵ��ԭ���ķ����Ͷಽ��Ӧ��ϵ�ļ���ȣ��Ѷ��еȣ���6����ע�����ù�ϵʽ���м��㣬Ϊ�״��㣬ѧ�������Եζ�����ȡ��Һ���������ϡ�ͺ���Һ������㣬���´���𰸣�

| A�� | 1mol�Ȼ�������ˮ���1 L��Һ��������Һ��Cl-�����ʵ���Ũ��Ϊ1mol/L | |
| B�� | �ڱ�״���£�11.2LH2O��������ԭ����ΪNA | |
| C�� | CO2��Ħ������Ϊ44g | |
| D�� | ��״����22.4LCH4��l8gH2O�����еĵ�������Ϊ10NA |
| A�� | ��Һ��Ϊ��ɫ | |
| B�� | ��Һ�������ݲ�������Һ���ձ�Ϊ��ɫ | |
| C�� | ������Һ��ɫ�������������� | |
| D�� | ��Һ���ձ�Ϊ��ɫ |
| A�� | Cu��OH��2������ Cu��OH��2����� | |
| B�� | BaCl2��Һ��Na2SO4��Һ Ba��OH��2��Һ��Na2SO4��Һ | |
| C�� | NaHCO3��Һ��NaHSO4��Һ Na2CO3��Һ��NaHSO4��Һ | |
| D�� | ʯ��ʯ������ ��ʯ�������� |
| A�� | 1.0L1.0mo1•L-1��NaNO3ˮ��Һ�к��е���ԭ����Ϊ3NA | |
| B�� | 3.9gNa2O2������������������Ϊ0.15NA | |
| C�� | 25��ʱpH=13��Ba��OH��2��Һ�к���OHһ����ĿΪ0.1NA | |
| D�� | 1 mol���ǻ���1 mol������������������������Ϊ9NA |
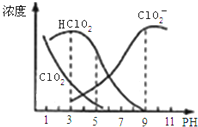 ����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������
����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������| A�� | �������������������½��ȶ� | |
| B�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ֵKa=10-6 | |
| C�� | pHԽ��Ư����Ư������Խ�� | |
| D�� | 25�棬pH=3ʱ��NaClO2��Һ�У�c��Na+��+c��H+��=c��ClO2-��+c��OH-�� |
 ��1����������������
��1����������������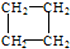 ��
��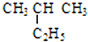 ������ ��C��CH3��4��
������ ��C��CH3��4�� �����л�Ϊͬ���칹����Ǣڢܣ�����ţ��������
�����л�Ϊͬ���칹����Ǣڢܣ�����ţ��������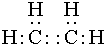 ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ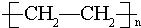 ��
�� ��֪��CH3CH2OH+NaBr+H2SO4��Ũ�� $\stackrel{��}{��}$ CH3CH2Br+NaHSO4+H2O��
��֪��CH3CH2OH+NaBr+H2SO4��Ũ�� $\stackrel{��}{��}$ CH3CH2Br+NaHSO4+H2O��