Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņłýĺ›Ő‚“‚ÕÍ≥…Ō¬Ń–ő Ő‚°£
£®1£©ĹęĶ»÷ ŃŅĶń–Ņ∑Ř∑÷ĪūÕ∂»Ž10 mL 0.1 mol°§L£≠1—őňŠļÕ10 mL 0.1 mol°§L£≠1 CH3COOH»‹“ļ÷–°£»Ű–Ņ≤Ľ◊„ŃŅ£¨∑ī”¶ŅžĶń «________£®ŐÓ–ī—őňŠ»‹“ļ°Ęī◊ňŠ»‹“ļ£©°£
£®2£©ĹęĶ»ŃŅĶń–Ņ∑Ř∑÷ĪūÕ∂»Žc£®H£ę£©ĺýő™1 mol°§L£≠1°ĘŐŚĽżĺýő™10 mLĶń—őňŠļÕī◊ňŠ»‹“ļ÷–°£»Ű–Ņ≤Ľ◊„ŃŅ£¨∑ī”¶ŅžĶń «________£®ŐÓ–ī—őňŠ»‹“ļ°Ęī◊ňŠ»‹“ļ£©°£
£®3£©…ŤňģĶńĶÁņŽ∆Ĺļ‚ŌŖ»ÁÕľňý ĺ°£

a.»Ű“‘AĶ„ĪŪ ĺ25°„ Īňģ‘ŕĶÁņŽ∆Ĺļ‚ ĪĶńŃ£◊”Ň®∂»£¨ĶĪő¬∂»…żłŖĶĹ100°„ Ī£¨ňģĶńĶÁņŽ∆Ĺļ‚◊īŐ¨ĶĹBĶ„£¨‘Úīň ĪňģĶńņŽ◊”Ľż‘Ųľ”ĶĹ____________£Ľ
b.ĹępH=8ĶńBa£®OH£©2»‹“ļ”ŽpH=5ĶńŌ°—őňŠĽžļŌ£¨≤ĘĪ£≥÷‘ŕ100°„Ķńļ„ő¬£¨”Ż ĻĽžļŌ»‹“ļĶńpH=7£¨‘ÚBa(OH) 2»‹“ļļÕ—őňŠĶńŐŚĽżĪ»ő™___________°£
£®4£©25°ś Ī£¨CH3COOH”ŽCH3COONaĶńĽžļŌ»‹“ļ£¨»Ű≤‚Ķ√ĽžļŌ»‹“ļpH£Ĺ6£¨‘Ú»‹“ļ÷–c£®CH3COO£≠£©£≠c£®Na£ę£©£Ĺ________ mol/L £®ŐÓ◊ľ»∑ ż÷Ķ£©°£
£®5£©“—÷™NaHSO4‘ŕňģ÷–ĶńĶÁņŽ∑Ĺ≥Ő Ĺő™NaHSO4=Na£ę£ęH£ę£ęSO42£≠°£ń≥ő¬∂»Ō¬£¨ŌÚc£®H£ę£©£Ĺ1°Ń10£≠6 mol°§L£≠1Ķń’ŰŃůňģ÷–ľ”»ŽNaHSO4ĺßŐŚ£¨Ī£≥÷ő¬∂»≤ĽĪš£¨≤‚Ķ√»‹“ļĶńc£®H£ę£©£Ĺ1°Ń10£≠2 mol°§L£≠1°£Ō¬Ń–∂‘ł√»‹“ļĶń–ū Ų’ż»∑Ķń «________________°£
A£ģł√ő¬∂»łŖ”ŕ25 °ś
B£ģ”…ňģĶÁņŽ≥ŲņīĶńH£ęĶńŇ®∂»ő™1°Ń10£≠10 mol°§L£≠1
C£ģ»°ł√»‹“ļľ”ňģŌ° Õ100Ī∂£¨»‹“ļ÷–ĶńňģĶÁņŽ≥ŲĶńc£®H£ę£©ľű–°
D£ģľ”»ŽNaHSO4ĺßŐŚ“÷÷∆ňģĶńĶÁņŽ
°ĺīūįł°Ņ—őňŠ»‹“ļ ī◊ňŠ»‹“ļ 10-12 2:9 99°Ń10£≠8mol/L ABD
°ĺĹ‚őŲ°Ņ
£®1£©łý囫‚ņŽ◊”Ň®∂»īů–°Ň–∂Ō£Ľ
£®2£©łý囫‚ņŽ◊”Ň®∂»īů–°Ň–∂Ō£Ľ
£®3£©łýĺ›c(H£ę)°Ęc(OH-)ĪšĽĮŇ–∂ŌKwĶńĪšĽĮ£Ľ
£®4£©łýĺ›ĶÁļ… ōļ„c(H£ę)+c(Na+)= c(OH-)+c(CH3COO-)ĹÝ––ľ∆ň„£Ľ
£®5£©łýĺ›ňģĶńĶÁņŽ∆Ĺļ‚ĹÝ––∑÷őŲ°£
£®1£©ŃĹ÷÷“Ľ‘™ňŠ»‹÷ ĶńőÔ÷ ĶńŃŅŌŗĶ»£¨Ķę—őňŠ÷–c(H£ę)īů£¨Ļ —őňŠ”Ž–Ņ∑ī”¶ĶńňŔ¬ Ņž£Ľ
£®2£©’‚ŃĹ÷÷ňŠĶńc(H£ę)ŌŗĶ»£¨Ō‘»Ľļů’ŖĶńőÔ÷ ĶńŃŅŇ®∂»īů£¨»‹÷ ĶńőÔ÷ ĶńŃŅ“≤īů£¨≥ű ľĶńňŔ¬ ŌŗÕ¨£¨Ķęňś◊Ň∑ī”¶ĶńĹÝ––£¨H£ę≤Ľ∂ŌĪĽŌŻļń£¨»űňŠCH3COOHĶńĶÁņŽ∆Ĺļ‚≤Ľ∂Ō’żŌÚ“∆∂Į£¨”÷ĶÁņŽ≥ŲH£ę£¨Ļ ‘ŕ∑ī”¶ĹÝ––ĶńÕ¨“Ľ ĪŅŐCH3COOH»‹“ļ÷–Ķńc(H£ę)īů”ŕ—őňŠ»‹“ļ÷–Ķńc(H£ę)£¨ňý“‘ī◊ňŠ”Ž–Ņ∑ī”¶ňŔ¬ Ņž£Ľ
£®3£©a.25°ś ĪīŅňģ÷–c(H£ę)=c(OH-)=10-7 mol/L£¨Kw= c(H£ę)c(OH-)=10-14£¨ĶĪő¬∂»…żłŖĶĹ100°ś£¨īŅňģ÷–c(H£ę)= c(OH-)=10-6 mol/L£¨‘ÚKw= c(H£ę)c(OH-)=10-12£¨ī”AĶ„ĶĹBĶ„£¨ňģĶńņŽ◊”Ľżī”10-14‘Ųľ”ĶĹ10-12£Ľ
b. 100°ś Ī£¨ĹępH=8ĶńBa(OH)2»‹“ļ÷–£ļc(OH-)=10-4 mol/L£¨pH=5ĶńŌ°—őňŠ÷–£ļc(H£ę)=10-5 mol/L£¨…Ť«‚—űĽĮĪĶĶńŐŚĽżő™x£¨—őňŠĶńŐŚĽżő™y£¨100°śĶńļ„ő¬£¨ĽžļŌ»‹“ļpH=7£¨»‹“ļ≥ ľÓ–‘£¨‘Ú»‹“ļ÷–«‚—űłýņŽ◊”Ň®∂»ő™£ļ![]() =10-5 mol/L£¨
=10-5 mol/L£¨
‘Ú£ļc(OH-)=![]() =10-5 mol/L£¨Ĺ‚Ķ√x£ļy=2£ļ9£Ľ
=10-5 mol/L£¨Ĺ‚Ķ√x£ļy=2£ļ9£Ľ
£®4£©25°ś Ī£¨CH3COOH”ŽCH3COONaĶńĽžļŌ»‹“ļ£¨»Ű≤‚Ķ√ĽžļŌ»‹“ļpH£Ĺ6£¨ĽžļŌ»‹“ļŌ‘ňŠ–‘£¨‘Úc(H£ę)= 10-6 mol/L£¨c(OH-)=10-8mol/L£¨»‹“ļ÷–ĶÁļ… ōļ„ő™c(H£ę)+c(Na+)= c(OH-)+c(CH3COO-)£¨‘Úc(CH3COO-)-c(Na+)= c(H£ę)- c(OH-)=10-6 mol/L -10-8mol/L=99°Ń10£≠8mol/L£Ľ
£®5£©A£ģ25°ś ĪīŅňģ÷–c(H£ę)=1°Ń10-7mol/L£¨c(H£ę)=1°Ń10-6mol/LňĶ√ųīŔĹÝŃňňģĶńĶÁņŽ£¨Ļ T>25°ś£¨—°ŌÓA’ż»∑£Ľ
B£ģpH=6£¨ňģĶńņŽ◊”Ľż≥£ żő™1°Ń10-12£¨ňģĶńņŽ◊”Ľż≥£ ż=«‚ņŽ◊””Ž«‚—űłýŇ®∂»Ķń≥ňĽż£¨»‹“ļĶńpHő™2£¨Ļ ”…ňģĶÁņŽ≥ŲņīĶńc(H£ę)=1°Ń10-10mol/L£¨—°ŌÓB’ż»∑£Ľ
C£ģő¬∂»≤ĽĪš Ī£¨Kw≤ĽĪš£¨ľ”ňģŌ° Õc(H£ę)ľű–°£¨Kw= c(H£ę)°Ńc(OH-)£¨ňý“‘c(OH-)‘Ųīů£¨—°ŌÓCīŪőů£Ľ
D£ģNaHSO4ĶńĶÁņŽ…ķ≥…«‚ņŽ◊”£¨∂‘ňģĶńĶÁņŽ∆ū“÷÷∆◊ų”√£¨ňģĶńĶÁņŽ≥Ő∂»ľű–°£¨—°ŌÓD’ż»∑£Ľ
īūįł—°ABD°£

°ĺŐ‚ńŅ°Ņ“—÷™≤‚∂®÷–ļÕ»»Ķń Ķ—ť≤Ĺ÷Ť»ÁŌ¬£ļ
![]() ŃŅ»°50mL
ŃŅ»°50mL![]() ŃÚňŠĶĻ»Ž–°…’Ī≠÷–£¨≤‚ŃŅő¬∂»£Ľ
ŃÚňŠĶĻ»Ž–°…’Ī≠÷–£¨≤‚ŃŅő¬∂»£Ľ
![]() ŃŅ»°50mL
ŃŅ»°50mL![]() NaOH»‹“ļ£¨≤‚ŃŅő¬∂»£Ľ
NaOH»‹“ļ£¨≤‚ŃŅő¬∂»£Ľ
![]() ĹęNaOH»‹“ļĶĻ»Ž–°…’Ī≠÷–£¨ĽžļŌĺý‘»ļů≤‚ŃŅĽžļŌ“ļő¬∂»£ģ
ĹęNaOH»‹“ļĶĻ»Ž–°…’Ī≠÷–£¨ĽžļŌĺý‘»ļů≤‚ŃŅĽžļŌ“ļő¬∂»£ģ
«ŽĽōīū£ļ
£®1£©![]() »‹“ļ…‘ĻżŃŅĶń‘≠“Ú______£ģ
»‹“ļ…‘ĻżŃŅĶń‘≠“Ú______£ģ
£®2£©ľ”»ŽNaOH»‹“ļĶń’ż»∑≤Ŕ◊ų «______![]() ŐÓ◊÷ńł
ŐÓ◊÷ńł![]() £ģ
£ģ
A.—ō≤£ŃßįŰĽļ¬żľ”»Ž![]() “Ľīő—łňŔľ”»Ž
“Ľīő—łňŔľ”»Ž![]() ∑÷»żīőľ”»Ž
∑÷»żīőľ”»Ž
£®3£© ĻŃÚňŠ”ŽNaOH»‹“ļĽžļŌĺý‘»Ķń’ż»∑≤Ŕ◊ų «______£ģ
ő¬∂» Ķ—ťīő ż | ∆ū ľő¬∂» | ÷’÷Ļő¬∂»
| ő¬∂»≤Ó∆Ĺĺý÷Ķ
| ||
| NaOH | ∆Ĺĺý÷Ķ | |||
1 |
|
|
|
|
|
2 |
|
|
|
|
|
3 |
|
|
|
|
|
£®4£©…Ť»‹“ļĶń√‹∂»ĺýő™![]() £¨÷–ļÕļů»‹“ļĶńĪ»»»»›
£¨÷–ļÕļů»‹“ļĶńĪ»»»»›![]() £¨«Žłýĺ› Ķ—ť żĺ›«ů≥Ų÷–ļÕ»»ő™______–ī≥Ųł√∑ī”¶Ķń»»ĽĮ—ß∑Ĺ≥Ő Ĺ______
£¨«Žłýĺ› Ķ—ť żĺ›«ů≥Ų÷–ļÕ»»ő™______–ī≥Ųł√∑ī”¶Ķń»»ĽĮ—ß∑Ĺ≥Ő Ĺ______
£®5£©»ŰĹęļ¨![]()
![]() ĶńŇ®ŃÚňŠ”Žļ¨1molNaOHĶń»‹“ļĽžļŌ£¨∑Ň≥ŲĶń»»ŃŅ______
ĶńŇ®ŃÚňŠ”Žļ¨1molNaOHĶń»‹“ļĽžļŌ£¨∑Ň≥ŲĶń»»ŃŅ______![]() ŐÓ°į–°”ŕ°Ī°Ę°įĶ»”ŕ°ĪĽÚ°įīů”ŕ°Ī
ŐÓ°į–°”ŕ°Ī°Ę°įĶ»”ŕ°ĪĽÚ°įīů”ŕ°Ī![]() £¨‘≠“Ú «______£ģ
£¨‘≠“Ú «______£ģ
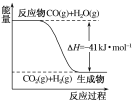
°ĺŐ‚ńŅ°ŅĻ§“Ķ…ķ≤ķŃÚňŠĹ”ī• “∑Ę…ķ»ÁŌ¬ĽĮ—ß∑ī”¶£ļ2SO2(g)£ęO2(g)![]() 2SO3(g) ¶§H£Ĺ£≠196.6kJ°§mol£≠1°£“Ľ∂®ő¬∂»Ō¬£¨ŌÚľ◊°Ę““°ĘĪŻ»żłŲ»›Ľżĺýő™2LĶńļ„»›√‹Ī’»›∆ų÷–Õ∂»ŽSO2(g)ļÕO2(g)£¨∆š∆ū ľőÔ÷ ĶńŃŅľįSO2Ķń∆Ĺļ‚◊™ĽĮ¬ »ÁĪŪňý ĺ£¨Ō¬Ń–Ň–∂Ō÷–£¨’ż»∑Ķń «£® £©
2SO3(g) ¶§H£Ĺ£≠196.6kJ°§mol£≠1°£“Ľ∂®ő¬∂»Ō¬£¨ŌÚľ◊°Ę““°ĘĪŻ»żłŲ»›Ľżĺýő™2LĶńļ„»›√‹Ī’»›∆ų÷–Õ∂»ŽSO2(g)ļÕO2(g)£¨∆š∆ū ľőÔ÷ ĶńŃŅľįSO2Ķń∆Ĺļ‚◊™ĽĮ¬ »ÁĪŪňý ĺ£¨Ō¬Ń–Ň–∂Ō÷–£¨’ż»∑Ķń «£® £©
ľ◊ | ““ | ĪŻ | ||
∆ū ľőÔ÷ ĶńŃŅ | n(SO2)/mol | 0.6 | 1.2 | 1.2 |
n(O2)/mol | 0.36 | 0.36 | 0.72 | |
SO2Ķń∆Ĺļ‚◊™ĽĮ¬ | 80% | ¶Ń1 | ¶Ń2 | |
A.ľ◊°ķ““£¨∆Ĺļ‚ŌÚ’ż∑ī”¶∑ĹŌÚ“∆∂Į£¨¶Ń(O2)‘Ųīů£¨∑Ň≥ŲĶń»»ŃŅő™47.18kJ
B.∆Ĺļ‚ Ī£¨ĪŻ÷–c(SO2) «ľ◊÷–Ķń2Ī∂
C.∆Ĺļ‚ Ī£¨SO2Ķń◊™ĽĮ¬ £ļ¶Ń2>80%>¶Ń1
D.ł√ő¬∂»Ō¬£¨∆Ĺļ‚≥£ żK£Ĺ400
°ĺŐ‚ńŅ°ŅŌ¬Ń–”–ĻōňńłŲ≥£”√ĶÁĽĮ—ß◊į÷√Ķń–ū Ų÷–£¨’ż»∑Ķń «![]() °°°°
°°°°![]()
|
|
|
|
ÕľĘŮľÓ–‘–Ņ√ŐĶÁ≥ō | ÕľĘÚ«¶–ÓĶÁ≥ō | ÕľĘůĶÁĹ‚ĺęŃ∂Õ≠ | ÕľĘŰ«‚—ű»ľŃŌĶÁ≥ō |
A. ÕľĘŮňý ĺĶÁ≥ō÷–£¨MnO2 «’żľę£¨ĶÁľę∑ī”¶ Ĺ «2H2O+2e-=H2°Ł+2OH-
B. ÕľĘÚňý ĺĶÁ≥ō∑ŇĶÁĻż≥Ő÷–£¨ĶĪÕ‚ĶÁ¬∑Õ®Ļż1molĶÁ◊” Ī£¨ņŪ¬Ř…ŌłļľęįŚĶń÷ ŃŅ‘Ųľ”96g
C. ÕľĘůňý ĺ◊į÷√Ļ§◊ųĻż≥Ő÷–£¨—Űľę÷ ŃŅľű…ŔŃŅĶ»”ŕ“űľęĶń÷ ŃŅ‘Ųľ”ŃŅ
D. ÕľĘŰňý ĺĶÁ≥ō÷–£¨≤ĽĻ‹KOH»‹“ļĽĽ≥…H2SO4»‹“ļĽĻ «Na2SO4»‹“ļ£¨ĶÁ≥ōĶń◊‹∑ī”¶ Ĺ≤ĽĪš