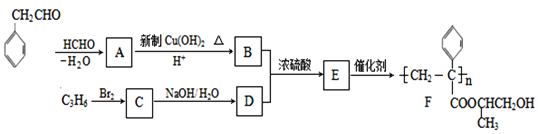
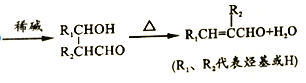ЬтФПФкШн
ЁОЬтФПЁПвбжЊВтЖЈжаКЭШШЕФЪЕбщВНжшШчЯТЃК
![]() СПШЁ50mL
СПШЁ50mL![]() СђЫсЕЙШыаЁЩеБжаЃЌВтСПЮТЖШЃЛ
СђЫсЕЙШыаЁЩеБжаЃЌВтСПЮТЖШЃЛ
![]() СПШЁ50mL
СПШЁ50mL![]() NaOHШмвКЃЌВтСПЮТЖШЃЛ
NaOHШмвКЃЌВтСПЮТЖШЃЛ
![]() НЋNaOHШмвКЕЙШыаЁЩеБжаЃЌЛьКЯОљдШКѓВтСПЛьКЯвКЮТЖШЃЎ
НЋNaOHШмвКЕЙШыаЁЩеБжаЃЌЛьКЯОљдШКѓВтСПЛьКЯвКЮТЖШЃЎ
ЧыЛиД№ЃК
ЃЈ1ЃЉ![]() ШмвКЩдЙ§СПЕФдвђ______ЃЎ
ШмвКЩдЙ§СПЕФдвђ______ЃЎ
ЃЈ2ЃЉМгШыNaOHШмвКЕФе§ШЗВйзїЪЧ______![]() ЬюзжФИ
ЬюзжФИ![]() ЃЎ
ЃЎ
A.биВЃСЇАєЛКТ§МгШы![]() вЛДЮбИЫйМгШы
вЛДЮбИЫйМгШы![]() ЗжШ§ДЮМгШы
ЗжШ§ДЮМгШы
ЃЈ3ЃЉЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЪЧ______ЃЎ
ЮТЖШ ЪЕбщДЮЪ§ | Ц№ЪМЮТЖШ | жежЙЮТЖШ
| ЮТЖШВюЦНОљжЕ
| ||
| NaOH | ЦНОљжЕ | |||
1 |
|
|
|
|
|
2 |
|
|
|
|
|
3 |
|
|
|
|
|
ЃЈ4ЃЉЩшШмвКЕФУмЖШОљЮЊ![]() ЃЌжаКЭКѓШмвКЕФБШШШШн
ЃЌжаКЭКѓШмвКЕФБШШШШн![]() ЃЌЧыИљОнЪЕбщЪ§ОнЧѓГіжаКЭШШЮЊ______аДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН______
ЃЌЧыИљОнЪЕбщЪ§ОнЧѓГіжаКЭШШЮЊ______аДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН______
ЃЈ5ЃЉШєНЋКЌ![]()
![]() ЕФХЈСђЫсгыКЌ1molNaOHЕФШмвКЛьКЯЃЌЗХГіЕФШШСП______
ЕФХЈСђЫсгыКЌ1molNaOHЕФШмвКЛьКЯЃЌЗХГіЕФШШСП______![]() ЬюЁАаЁгкЁБЁЂЁАЕШгкЁБЛђЁАДѓгкЁБ
ЬюЁАаЁгкЁБЁЂЁАЕШгкЁБЛђЁАДѓгкЁБ![]() ЃЌдвђЪЧ______ЃЎ
ЃЌдвђЪЧ______ЃЎ
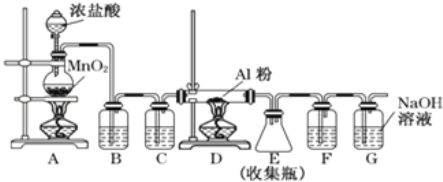
ЁОД№АИЁПШЗБЃСђЫсБЛЭъШЋжаКЭ ![]() гУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏ
гУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏ ![]()
![]() Дѓгк ХЈСђЫсШмгкЫЎЗХГіШШСП
Дѓгк ХЈСђЫсШмгкЫЎЗХГіШШСП
ЁОНтЮіЁП
ЃЈ1ЃЉЮЊСЫШЗБЃЖЈСПЕФСђЫсЗДгІЭъШЋЃЌЫљгУNaOHЩдЙ§СПЃЛ
ЃЈ2ЃЉНЋNaOHШмвКЕЙШыаЁЩеБжаЃЌВЛФмЗжМИДЮЕЙШыЃЌЗёдђЛсЕМжТШШСПЩЂЪЇЃЌгАЯьВтЖЈНсЙћЃЛ
ЃЈ3ЃЉСђЫсКЭЧтбѕЛЏФЦЛьКЯЪБЃЌгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏЃЌЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЃЛ
ЃЈ4ЃЉЯШИљОнQ=mcЁїTМЦЫуЗДгІЗХГіЕФШШСПЃЌШЛКѓИљОнЁїH=-Q/nkJ/molМЦЫуГіЗДгІШШЃЛИљОнШШЛЏбЇЗНГЬЪНЕФЪщаДЗНЗЈРДЪщаДЃЎ
ЃЈ5ЃЉХЈСђЫсШмгкЫЎЛсЗХШШЁЃ
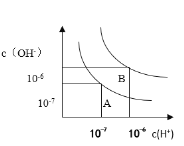
![]() ЪЕбщжаЃЌЫљгУNaOHЩдЙ§СПЕФдвђЪЧШЗБЃСђЫсЗДгІЭъШЋЃЛ
ЪЕбщжаЃЌЫљгУNaOHЩдЙ§СПЕФдвђЪЧШЗБЃСђЫсЗДгІЭъШЋЃЛ
ЙЪД№АИЮЊЃКШЗБЃСђЫсБЛЭъШЋжаКЭЃЛ
![]() ЕЙШыЧтбѕЛЏФЦШмвКЪБЃЌБиаывЛДЮбИЫйЕФЕЙШыЃЌФПЕФЪЧМѕЩйШШСПЕФЩЂЪЇЃЌВЛФмЗжМИДЮЕЙШыЧтбѕЛЏФЦШмвКЃЌЗёдђЛсЕМжТШШСПЩЂЪЇЃЌгАЯьВтЖЈНсЙћЃЛ
ЕЙШыЧтбѕЛЏФЦШмвКЪБЃЌБиаывЛДЮбИЫйЕФЕЙШыЃЌФПЕФЪЧМѕЩйШШСПЕФЩЂЪЇЃЌВЛФмЗжМИДЮЕЙШыЧтбѕЛЏФЦШмвКЃЌЗёдђЛсЕМжТШШСПЩЂЪЇЃЌгАЯьВтЖЈНсЙћЃЛ
ЙЪбЁЃКBЃЛ
![]() ЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЗНЗЈЪЧЃКгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏЃЛ
ЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЗНЗЈЪЧЃКгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏЃЛ
ЙЪД№АИЮЊЃКгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏЃЛ
![]() СђЫсгы
СђЫсгы![]() ШмвКНјаажаКЭЗДгІЩњГЩЫЎЕФЮяжЪЕФСПЮЊ
ШмвКНјаажаКЭЗДгІЩњГЩЫЎЕФЮяжЪЕФСПЮЊ![]() ЃЌШмвКЕФжЪСПЮЊ
ЃЌШмвКЕФжЪСПЮЊ![]() ЃЌЮТЖШБфЛЏЕФжЕЮЊШ§ДЮЪЕбщЕФЦНОљжЕ
ЃЌЮТЖШБфЛЏЕФжЕЮЊШ§ДЮЪЕбщЕФЦНОљжЕ![]() ЃЌдђЩњГЩ
ЃЌдђЩњГЩ![]() ЫЎЗХГіЕФШШСПЮЊ
ЫЎЗХГіЕФШШСПЮЊ![]() ЃЌМД
ЃЌМД![]() ЃЌ
ЃЌ
ЫљвдЪЕбщВтЕУЕФжаКЭШШ![]() ЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪН
ЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪН![]() ЃЛ
ЃЛ
ЙЪД№АИЮЊЃК![]() ЃЛ
ЃЛ![]() ЃЛ
ЃЛ
![]() ХЈСђЫсШмгкЫЎЗХГіШШСПЃЌЫљвдКЌ
ХЈСђЫсШмгкЫЎЗХГіШШСПЃЌЫљвдКЌ![]() ЕФХЈСђЫсгыКЌ
ЕФХЈСђЫсгыКЌ![]() ЕФШмвКЛьКЯЃЌЗХГіЕФШШСПДѓгк
ЕФШмвКЛьКЯЃЌЗХГіЕФШШСПДѓгк![]() ЁЃ
ЁЃ
ЙЪД№АИЮЊЃКДѓгкЃЛХЈСђЫсШмгкЫЎЗХГіШШСПЁЃ

ЁОЬтФПЁПвбжЊ1gЧтЦјЭъШЋШМЩеЩњГЩвКЬЌЫЎЪБЗХГіШШСП143kJЃЌ18gЫЎеєЦјБфГЩвКЬЌЫЎЗХГі44kJЕФШШСПЁЃЦфЫћЯрЙиЪ§ОнШчЯТБэЃК
ЛЏбЇМќ | O=O | HЁЊH | HЁЊO(g) |
1 molЛЏбЇМќЖЯСбЪБ ашвЊЮќЪеЕФФмСП/kJ | 496 | x | 463 |
дђБэжаxЮЊЃЈ ЃЉ
A.920B.557C.436D.188