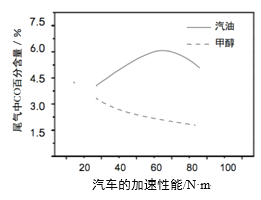
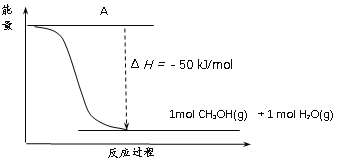
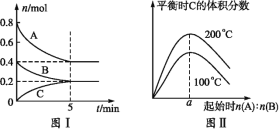
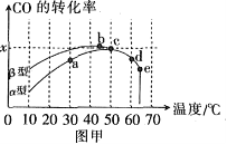
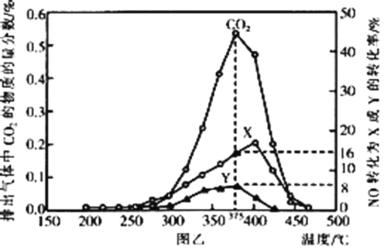
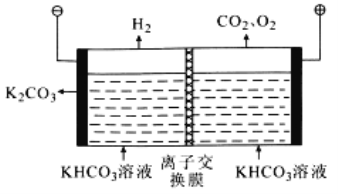
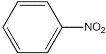
��Ŀ����
����Ŀ��������ʵ����ʵ���ó�����Ӧ���۲���ȷ����( )
ѡ�� | ʵ����ʵ | ���� |
A | ������������,0.01mol��L-1������KMnO4��Һ�ֱ���0.1mol��L-1��H2C2O4��Һ��0.2mol��L-1��H2C2O4��Һ��Ӧ,������ɫʱ��� | ��������������ʱ,����Ӧ��Ũ�ȿ���ʹ��ѧ��Ӧ���ʼӿ� |
B | ������������,�ֱ�����������ʵ���Ũ�ȵ�Na2S2O3��Һ��������Һ�Ļ��Һ������ˮ����ˮ��,������ˮ�еĻ��Һ�ȳ��ֻ��� | ��������������ʱ,��Ӧ��ϵ���¶�Խ��,��ѧ��Ӧ����Խ�� |
C | һ��������,�ֱ����ݻ�Ϊ1L���ݻ�Ϊ2L�������ܱ������м�������������͵�����,��ͬ�¶��·������·�Ӧ:H2(g)+I2(g) | ��������������ʱ,����̬��Ӧ��ϵ��ѹǿԽ��,��ѧ��Ӧ����Խ�� |
D | ������MnO2��ĩ����ʢ��10%H2O2��Һ����ƿ��,�ڻ�ѧ��Ӧǰ��,MnO2�������ͻ�ѧ���ʶ�û�з����仯 | ������Ȼ���Լӿ컯ѧ��Ӧ������,��һ�������뻯ѧ��Ӧ |
A.AB.BC.CD.D
���𰸡�D
��������
A��������������,0.01mol��L-1������KMnO4��Һ�ֱ���0.1mol��L-1��H2C2O4��Һ��0.2mol��L-1��H2C2O4��Һ��Ӧ,������ɫʱ��̡��Ӷ��ó����ۣ���������������ʱ,����Ӧ��Ũ�ȿ���ʹ��ѧ��Ӧ���ʼӿ졣������ȷ��
B��������������,�ֱ�����������ʵ���Ũ�ȵ�Na2S2O3��Һ��������Һ�Ļ��Һ������ˮ����ˮ��,������ˮ�еĻ��Һ�ȳ��ֻ��ǡ��Ӷ��ó����ۣ���������������ʱ,��Ӧ��ϵ���¶�Խ��,��ѧ��Ӧ����Խ�졣������ȷ��
C��һ��������,�ֱ����ݻ�Ϊ1L���ݻ�Ϊ2L�������ܱ������м�������������͵�����,��ͬ�¶��·������·�Ӧ:H2(g)+I2(g)![]() 2HI(g),��õ���HI(g)ʱǰ����Ҫ��ʱ���١��Ӷ��ó����ۣ���������������ʱ,����̬��Ӧ��ϵ��ѹǿԽ��,��ѧ��Ӧ����Խ�졣������ȷ��
2HI(g),��õ���HI(g)ʱǰ����Ҫ��ʱ���١��Ӷ��ó����ۣ���������������ʱ,����̬��Ӧ��ϵ��ѹǿԽ��,��ѧ��Ӧ����Խ�졣������ȷ��
D��������MnO2��ĩ����ʢ��10%H2O2��Һ����ƿ��,�ڻ�ѧ��Ӧǰ��,MnO2�������ͻ�ѧ���ʶ�û�з����仯���Ӷ��ó����ۣ�������Ȼ���Լӿ컯ѧ��Ӧ����,����һ�����뻯ѧ��Ӧ��������һ�������뷴Ӧ�����۴���
�ʴ�ΪD��

����Ŀ����֪A(g)��B(g) ![]() C(g)��D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)��D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
�¶�/�� | 700 | 900 | 830 | 1000 | 1200 |
ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
�ش��������⣺
(1)�÷�Ӧ�� ��H________0(�<���� >���� ����)��
(2)830��ʱ����һ��5 L���ܱ������г���0.20 mol��A��0.80 mol��B�� ��Ӧ��һ��ʱ��ﵽƽ��ʱA��ת����Ϊ________�������ʱ����ܱ��������ٳ���1 mol������ٴδ�ƽ��ʱA��ת���� ________�����������С�����䡱����
(3)����ѡ�����Ϊ�жϸ÷�Ӧ�ﵽƽ���������______��
a��ѹǿ����ʱ��ı� b��������ܶȲ���ʱ��ı�
c��c(A)����ʱ��ı� d����λʱ��������C��D�����ʵ������
(4)1200 ��ʱ��ӦC(g)��D(g) ![]() A(g)��B(g)��ƽ�ⳣ����ֵΪ________��
A(g)��B(g)��ƽ�ⳣ����ֵΪ________��