��Ŀ����
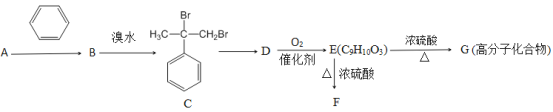
����Ŀ��һ�ִӷ�������﮵缫���ϣۺ�Li4Ti5O12��������̼�ۡ�PVDF����ƫ������ϩ�����л���ijЩ��Դ���������£�
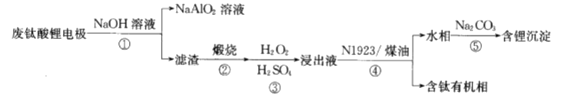
��֪��Li4Ti5O12+7H2SO4+5H2O2=2Li2SO4+5��TiO��H2O2����SO4+7H2O����������������ǣ���
A.������Ŀ���dz�ȥ̼����PVDF
B.����H2O2��������������
C.�Ӹõ缫�����пɻ��յĽ���Ԫ����Al��Ti��Li
D.���������Ͼɵ�أ������ڻ�����������Դ������
���𰸡�B
��������
A. ����̼����PVDF������������Ӧת��Ϊ�ӷ����������ȥ��A����ȷ��
B. ����֪��֪������û��Ԫ�ػ��ϼ۷����仯����Ӧ�Ƿ�������ԭ��Ӧ��B�����
C. ������ͼ�е���NaAlO2��������﮳��������������л�������֪�Ӹõ缫�����пɻ��յĽ���Ԫ����Al��Ti��Li��C����ȷ��
D. ���������Ͼɵ�أ������ڻ�����������Դ�����ã�D����ȷ��
��ѡB��

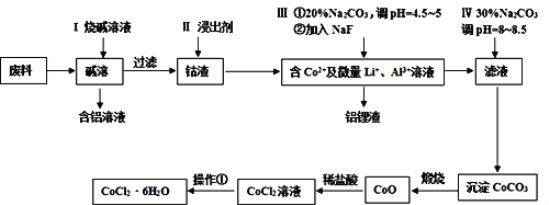
����Ŀ������������[CH3CH(OH)COO]2Fe3H2O��Mr=288����һ��ʳ�õIJ�����������Ч���������ã�������ˮ�������������Ҵ��������ֽ⣬��ͨ��������̼��������Ӧ�Ƶá�
CH3CH(OH)COOH+FeCO3+2H2O=[CH3CH(OH)COO]2Fe3H2O+CO2��
FeCO3������ˮ���ױ�������4FeCO3+6H2O+O2=4Fe(OH)3+4CO2
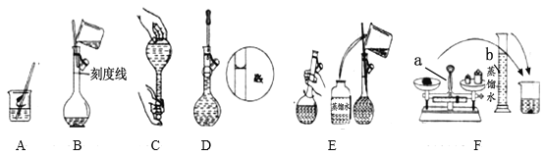
��.�����������Ʊ���
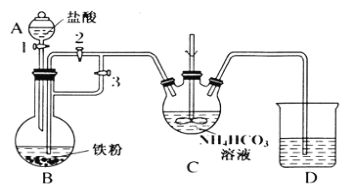
ij��ȤС����FeCl2��NH4HCO3�Ʊ�FeCO3��װ��ʾ��ͼ��ͼ��
�ش��������⣺
(1)Cװ�����漰����Ҫ��Ӧ�����ӷ���ʽ_________��
(2)��D�������崿�������ɵ�FeCl2��Һ��NH4HCO3��Һ���ʱ�IJ�����_____��
(3)���Ƶõ�FeCO3���뵽����������Һ�У��ټ����������ۣ�75���½��跴Ӧ�������������۵�������_______��
(4)��Ӧ������������ˣ���ȥ�������۵ķ�����_________��
(5)��������Һ�л��������������ķ����ǣ�________����ȴ�ᾧ�����ˣ� �������Ҵ�ϴ�ӣ����
��.�����������崿�ȵIJ�����
(6)����ȤС����KMnO4�ζ����ⶨ��Ʒ�������������������Ʒ�������������������������ֲ�Ʒ�������������Ǵ���100%����ԭ�������___��
(7)������������ȤС�������(Ce)�����ⶨ��Ʒ��Fe2+�ĺ������ζ���Ӧ���£�Ce4++Fe2+=Ce3++Fe3+��ȡ1.440g��Ʒ���100mL��Һ��ÿ��ȡ20.00mL�����б�Ҫ��������0.0500molL-1Ce(SO4)2����Һ�ζ����յ㣬��¼���������
�ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
1 | 0.20 | 19.95 |
2 | 0.10 | 21.65 |
3 | 0.95 | 20.60 |
���Ʒ��������������������Ϊ________%��(С�������һλ����)
(8)�����ʵ��֤���㹺������������������к�Fe2+��_______��
����Ŀ����.��п��ijŨ�ȵ����ᷴӦ��ʵ���У�ʵ��Ա�õ�����Ľ����
п��������g�� | п����״ | �¶ȣ��棩 | �ܽ����Ứ��ʱ�䣨s�� | |
A | 2 | ��Ƭ | 5 | 400 |
B | 2 | ��Ƭ | 15 | 200 |
C | 2 | ��Ƭ | 25 | 100 |
D | 2 | ��Ƭ | 35 | 50 |
E | 2 | ��Ƭ | 45 | 25 |
F | 2 | ��ĩ | 15 | 5 |
��1��д��ʵ���з�����Ӧ����������ʽ��___________���÷�Ӧ��_____��Ӧ��������������������������
��2����ϸ�۲�A~F��ʵ�����ݶԱȣ�����Եõ������¶�Ӱ�췴Ӧ���ʽ��ۣ��ô˽��ۣ����㣺55��ʱ��2gп��Ƭ�ܽ��������軨____ s��
��3���ԱȽ��B��F�����ͽ��F��ô���ԭ��________
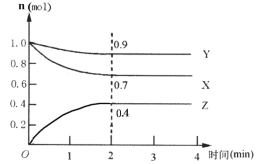
��.ij���淴Ӧ��ij���Ϊ5L���ܱ������н��У��ڴ�0~4���Ӹ����ʵ����ı仯�����ͼ��ʾ��X��Y��Z��Ϊ���壩��
��1���÷�Ӧ�ĵĻ�ѧ����ʽΪ_______��
��2����Ӧ��ʼ��2����ʱ��X��ƽ����Ӧ����Ϊ______��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����______��
a��ÿ����3molX��ͬʱ����1molY
b��������ѹǿ���ֲ���
c��Z��Ũ�Ȳ��ٱ仯
d�������ڻ��������ܶȱ��ֲ���
��4������ͼ���ƽ��ʱY��ת����Ϊ_____��