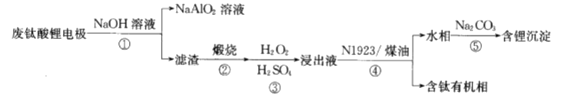
��Ŀ����
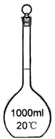
����Ŀ��������ij��ѧ����С������500mL3.5mol��L��1NaOH��Һ�IJ�����
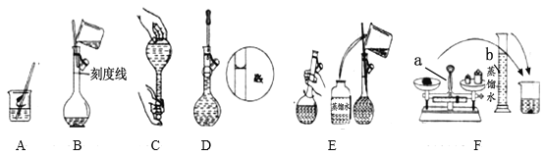
��1����д���������������ƣ�����a___������b ___��
��2��������Һʱ����������ƿ�ϱ��е�����Ϊ___��
��3�������ƹ����У������������������___��
��4������ƿʹ��ǰ��Ҫ�����Ƿ�©ˮ�������Ƿ�©ˮ��ʵ�����Ϊ___��
��5�����㰴������һ�����ʵ���Ũ����Һ�ľ�������ų�������˳��
���ϲ����У���ȷ�IJ�������˳��ΪF�� �� �� �� �� (�����)��___��
��6������������ͼʾ�д��ڴ������___(����ţ��ô�д��ĸ)��
���𰸡�������ƽ ��Ͳ 20�棬500 mL ��������ܽ⣬���� ������ƿ�м�ˮ���������ӣ����ÿ��Ƿ�©ˮ��Ȼ������ת����180�����ٵ��ÿ��Ƿ�©ˮ AEBDC DF
��������
��1�����������ƣ�����a��������ƽ������b����Ͳ��
��2��������Һʱ����������ƿ�ϱ��е�����Ϊ�¶Ⱥ��
��3�������ƹ����У��ܽ��ת��ʱ����ʹ�ò�������ǰ�ߵ������Ǽ����ܽ⣬���ߵ�������������
��4������ƿʹ��ǰ��Ҫ�����Ƿ�©ˮ�������Ƿ�©ˮ��ʵ�����Ϊ��ˮ�����������ã���������ת180�����ټ��������á�
��5������һ�����ʵ���Ũ����Һ�ľ����������Ϊ���㡢�������ܽ⡢ת�� �����ݣ���ͼ�п��Կ�����B��E�������Ⱥ��ǽ���Ĺؼ�����ͼ�п��Կ�����E������������ƿ�IJ���������ת��ʱ�IJ�������B�Ƕ���ʱ�IJ������ۺ�����������F��AΪ�������ܽ������EΪת�Ʋ�����B��C��DΪ���ݲ�����
��6����������ͼʾ�Ĵ���D�н�ͷ�ιܲ�������ƿ�У�F�г���ʱ��NaOH����ֱ�ӷ��������ϡ�
��1������a��������ƽ������b����Ͳ����Ϊ��������ƽ����Ͳ��
��2��������Һʱ����������ƿ�ϱ��е�����Ϊ20�棬500 mL����Ϊ��20�棬500 mL��
��3�������ƹ����У��ܽ��ת��ʱ����ʹ�ò�����������������������н�������ܽ⣬��������Ϊ����������ܽ⣬������
��4�������Ƿ�©ˮ��ʵ�����Ϊ��ˮ�����������ã���������ת180�����ٸ��������á���Ϊ��������ƿ�м�ˮ���������ӣ����ÿ��Ƿ�©ˮ��Ȼ������ת����180�����ٵ��ÿ��Ƿ�©ˮ��
��5������һ�����ʵ���Ũ����Һ�ľ����������Ϊ���㡢�������ܽ⡢ת�� �����ݣ���ͼ�п��Կ�����B��E�������Ⱥ��ǽ���Ĺؼ�����ͼ�п��Կ�����E������������ƿ�IJ���������ת��ʱ�IJ�������B�Ƕ���ʱ�IJ�������ȷ�IJ�������˳��ΪAEBDC����Ϊ��AEBDC��
��6����������ͼʾ�Ĵ���D�н�ͷ�ιܲ�������ƿ�У�F�г���ʱ��NaOH����ֱ�ӷ��������ϡ���Ϊ��DF��

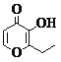
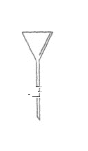
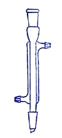
����Ŀ��I.�����Ǽ���ʵ���г��õ�������
|
|
|
|
A | B | C | D |
д����������������������ƣ�B___________��C___________��D___________��
II.��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݣ��ش��������⣺

��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________mol��L-1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����_____________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ
B������ƿ��ˮϴ�������ô�����Һϴ��
C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
D��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��4��ijѧ����������Ũ���������ˮ����490 mL���ʵ���Ũ��Ϊ0.400 mol��L-1��ϡ���ᡣ
����ѧ����Ҫ��ȡ________mL����Ũ����������ơ�������С�����1λ��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ��(�ں���������ƫ��������ƫС��������Ӱ����)��
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�档________________
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ��________________
����ͬѧȡ�������Ƶ�0.400 mol��L-1������ȥ�кͺ�0.4 g NaOH��NaOH��Һ�����������ȡ�����������������25mLҪС������ܵ�ԭ����________
A��Ũ����ӷ���Ũ�Ȳ���
B��������Һʱ��δϴ���ձ�
C��������Һʱ����������ƿ�̶���
D����ˮʱ�����̶��ߣ��ý�ͷ�ι�����
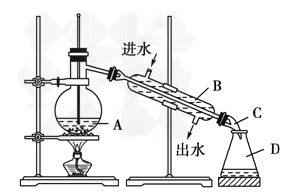
��5��ʵ��������õ�����ˮ������ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ�������ͼ�е��������ԵĴ���________________��________________��ʵ��ʱA�г�������������ˮ�⣬��������������Ƭ����������__________��
����Ŀ����ͼ��ʾ���������������ʵ�顣ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ����������һ������������档
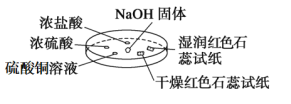
�±��ж�ʵ�����������Ľ��Ͳ���ȷ���ǣ� ��
ѡ�� | A | B | C | D |
ʵ�� ���� | Ũ���ḽ���������� | Ũ���ḽ������������ | �����ɫʯ����ֽ����ɫ��ʪ���ɫʯ����ֽ���� | ����ͭ��Һ����� |
���� | NH3��HCl��Ӧ������NH4Cl���� | NH3��Ũ���������Ӧ | NH3��ˮ��Ӧ������NH3��H2O�������OH��ʹ��ɫʯ����ֽ���� | ����Cu(OH)2���� |
A.AB.BC.CD.D