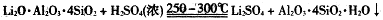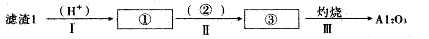��Ŀ����
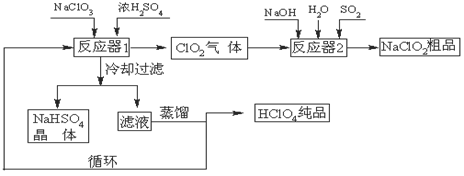
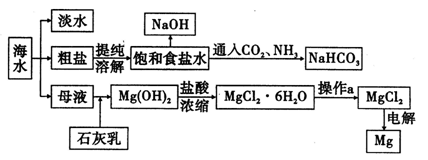
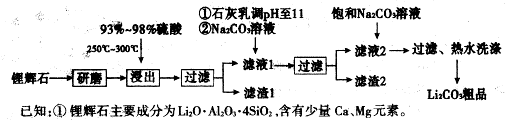
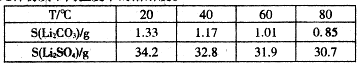
���������Ҫ�ɷ�ΪFeTiO3���ɱ�ʾΪFeO��TiO2������������MgO��CaO��SiO2�����ʡ������������Ʊ�����ӵ�ص缫���ϣ������Li4Ti5O12�����������LiFePO4���Ĺ�ҵ��������ͼ��ʾ��

��֪��FeTiO3�����ᷴӦ�����ӷ���ʽΪ��FeTiO3��4H+��4Cl-��Fe2+��TiOCl42-��2H2O
��1��������FeTiO3����Ԫ�صĻ��ϼ��� ��
��2������A�ijɷ��� ��
��3����ҺB��TiOCl42- ת������TiO2�����ӷ���ʽ�� ��
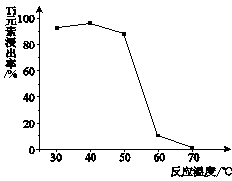
��4����Ӧ���й���TiO2ת����(NH4)2Ti5O15��Һʱ��TiԪ�صĽ������뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ����Ӧ�¶ȹ���ʱ��TiԪ�ؽ������½���ԭ���� ��
��5����Ӧ�۵Ļ�ѧ����ʽ�� ��
��6������ҺD�Ʊ�LiFePO4�Ĺ����У�����17%˫��ˮ��H2C2O4���������� ��
��7������������ﮣ�Li4Ti5O12������������ﮣ�LiFePO4�����缫��ɵ�أ��乤��ԭ��Ϊ��Li4Ti5O12��3LiFePO4 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4
�õ�س��ʱ������Ӧʽ�� ��

��֪��FeTiO3�����ᷴӦ�����ӷ���ʽΪ��FeTiO3��4H+��4Cl-��Fe2+��TiOCl42-��2H2O
��1��������FeTiO3����Ԫ�صĻ��ϼ��� ��
��2������A�ijɷ��� ��
��3����ҺB��TiOCl42- ת������TiO2�����ӷ���ʽ�� ��
��4����Ӧ���й���TiO2ת����(NH4)2Ti5O15��Һʱ��TiԪ�صĽ������뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ����Ӧ�¶ȹ���ʱ��TiԪ�ؽ������½���ԭ���� ��

��5����Ӧ�۵Ļ�ѧ����ʽ�� ��
��6������ҺD�Ʊ�LiFePO4�Ĺ����У�����17%˫��ˮ��H2C2O4���������� ��
��7������������ﮣ�Li4Ti5O12������������ﮣ�LiFePO4�����缫��ɵ�أ��乤��ԭ��Ϊ��Li4Ti5O12��3LiFePO4
 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4 �õ�س��ʱ������Ӧʽ�� ��
��12�֣�ÿ��2�֣�
��1��+2
��2��SiO2
��3��TiOCl42-��H2O TiO2����2H+��4Cl-
TiO2����2H+��4Cl-
��4���¶ȹ���ʱ����Ӧ�ﰱˮ����˫��ˮ�������ֽ�
��5�� (NH4)2Ti5O15��2LiOH��Li2Ti5O15����2NH3��H2O����2NH3��2H2O��
��6��20/9��1�֣�
��7��LiFePO4 �C e-��FePO4��Li+��1�֣�
��1��+2
��2��SiO2
��3��TiOCl42-��H2O
 TiO2����2H+��4Cl-
TiO2����2H+��4Cl-��4���¶ȹ���ʱ����Ӧ�ﰱˮ����˫��ˮ�������ֽ�
��5�� (NH4)2Ti5O15��2LiOH��Li2Ti5O15����2NH3��H2O����2NH3��2H2O��
��6��20/9��1�֣�
��7��LiFePO4 �C e-��FePO4��Li+��1�֣�
�����������1��������Ŀ������Ϣ���ɱ�ʾΪFeO��TiO2����֪Fe�Ļ��ϼ�Ϊ��+2��
��2��FeTiO3��MgO��CaO��HCl��Ӧ��ʣ�µĹ���ֻ��SiO2��
��3�����˺�δ��������Ӧ�����TiOCl42-��H2O��Ӧ������TiO2��ͬʱ����H+��Cl?��
��4��TiOCl42- ת��ΪTiO2ʱ����Ҫ���뷴Ӧ��˫��ˮ����ˮ�����������������ֽ⡣
��5��(NH4)2Ti5O15Ϊ��Σ�LiOHΪǿ��������ֽⷴӦ�����ݷ�Ӧ���ɣ�����д����ѧ����ʽ��
��6��������Ӧ���̣�H2O2��Fe2+����ΪFe3+��H2C2O4��Fe3+��ԭΪFe2+��H2O2��O��-1�۱�Ϊ-2�ۣ�H2C2O4��+3�۱�Ϊ+4�ۣ����ݵ���ת��������ȵã�m(H2O2)��17��200g/mol��2=m(H2C2O4)��90g/mol��2����m(H2O2)��m(H2C2O4)= 20/9��
��7����س��ʱ������ӦΪʧ���ӷ�Ӧ��LiFePO4��Feʧȥ1�����ӣ���+2�۱�Ϊ+3�ۣ�����FePO4��ͬʱ�õ�Li+��

��ϰ��ϵ�д�
�����Ŀ
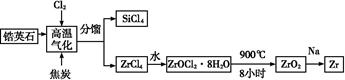
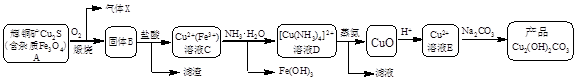
 Cu2+(aq) + 4NH3(aq)�������Ϲ������̣�����˵������ȷ����
Cu2+(aq) + 4NH3(aq)�������Ϲ������̣�����˵������ȷ���� CuO + 2HCl��+ 4NH3��
CuO + 2HCl��+ 4NH3��