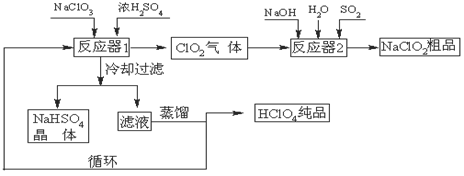
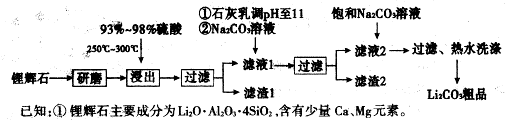
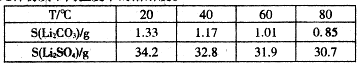
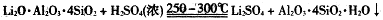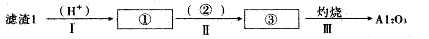��Ŀ����
��11�֣���.���ж���ʯ�ͣ��й���ϡ������ϡ���������ҹ�ս������Դ��Ӧ���Ա�����ϡ�����������ڱ��Т�B�����֡��ƺ���ϵʮ����Ԫ�ص��ܳƣ����Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�ۡ��ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ��ҹ��̲��ŷḻ���ƿ�ʯ��Y2FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�
��֪�����йؽ��������γ������������ʱ��pH���±���
�������ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
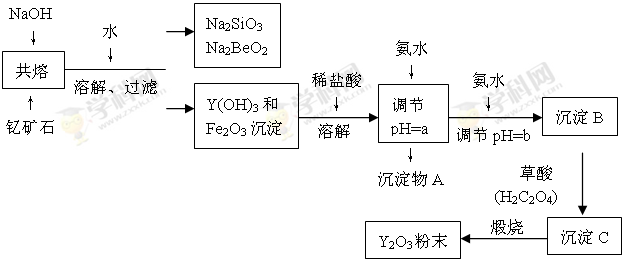
��1������Na2SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2�����������ѡ������� ������ĸ��
�����Լ���ͨ����Ҫ�IJ�������ʵ�֡�
A��NaOH��Һ B����ˮ C��CO2 D��HNO3
��2������ͼ���ð�ˮ����pH��aʱ���ɳ����Ļ�ѧʽΪ �������Ӱ�ˮ����pH��b����ʱ������Ӧ�����ӷ���ʽΪ ��
��3������CΪ�����ƣ�д�������Ƹ���������������Y2O3�Ļ�ѧ����ʽ�� ��
����ϵԪ���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�أ��ڼ��ȵ�������CeCl3����ˮ�⡣
��4����ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������NH4Cl��������___
��
��5����ijǿ���Ի��ϡ����Һ�м���H2O2������pH��3��Ce3+ͨ����Ӧ�γ�Ce(OH)4�������Է��룬 д����Ӧ�����ӷ���ʽ ��

��֪�����йؽ��������γ������������ʱ��pH���±���
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2.7 | 3.7 |
| Y3+ | 6.0 | 8.2 |
�������ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
��1������Na2SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2�����������ѡ������� ������ĸ��
�����Լ���ͨ����Ҫ�IJ�������ʵ�֡�
A��NaOH��Һ B����ˮ C��CO2 D��HNO3
��2������ͼ���ð�ˮ����pH��aʱ���ɳ����Ļ�ѧʽΪ �������Ӱ�ˮ����pH��b����ʱ������Ӧ�����ӷ���ʽΪ ��
��3������CΪ�����ƣ�д�������Ƹ���������������Y2O3�Ļ�ѧ����ʽ�� ��
����ϵԪ���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�أ��ڼ��ȵ�������CeCl3����ˮ�⡣
��4����ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������NH4Cl��������___
��
��5����ijǿ���Ի��ϡ����Һ�м���H2O2������pH��3��Ce3+ͨ����Ӧ�γ�Ce(OH)4�������Է��룬 д����Ӧ�����ӷ���ʽ ��
��11�֣���1��B ��1�֣�
��2��Fe(OH)3 ��1�֣���Y3++3NH3��H2O��Y(OH)3��+3NH4+ ��2�֣�
��3��Y2(C2O4)3 Y2O3+3CO2��+ 3CO�� ��3��)
Y2O3+3CO2��+ 3CO�� ��3��)
��4��NH4Cl�������ȷֽ����HCl������CeCl3ˮ�⣨2�֣�
��5��2Ce3++H2O2+6H2O��2Ce(OH)4��+ 6H+ ��2�֣�
��2��Fe(OH)3 ��1�֣���Y3++3NH3��H2O��Y(OH)3��+3NH4+ ��2�֣�
��3��Y2(C2O4)3
 Y2O3+3CO2��+ 3CO�� ��3��)
Y2O3+3CO2��+ 3CO�� ��3��)��4��NH4Cl�������ȷֽ����HCl������CeCl3ˮ�⣨2�֣�
��5��2Ce3++H2O2+6H2O��2Ce(OH)4��+ 6H+ ��2�֣�
�����������1�����ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�����Na2SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2�����������Na2BeO2�����ʺ�NaAlO2����ƶϣ��ӹ��������ᣬ�����Ʒ�Ӧ���ɹ��������Na2BeO2�ķ�Ӧ�����Ȼ�����Һ���ټ��������ˮ���������ӣ��ʴ�Ϊ��B��
��2����������ͼ��֪��������AӦ����������������������ͼ���ð�ˮ����pH��aʱ���ɳ����Ļ�ѧʽΪFe(OH)3�������йؽ��������γ������������ʱ��pH��֪�������Ӱ�ˮ����pH��b����ʱ���ɷֳ���Ӧ����Y(OH)3��������Ӧ�����ӷ���ʽΪY3++3NH3��H2O��Y(OH)3��+3NH4+��
��3������CΪ�����ƣ�����ԭ���غ��֪�������Ƹ���������������Y2O3��ͬʱ������CO��CO2���ɣ����Էֽ�Ļ�ѧ����ʽΪY2(C2O4)3
 Y2O3+3CO2��+ 3CO����
Y2O3+3CO2��+ 3CO������4���ڼ��ȵ�������CeCl3����ˮ�������Ȼ����Ce(OH)3������ڼ���ʱ�����ֹ��ˮ�⡣����NH4Cl�������ȷֽ����HCl��HCl��������CeCl3ˮ�⡣
��5��H2O2����ǿ�����ԣ�����Һ��ͨ������pH��3�����Խ�Ce3+�����γ�Ce(OH)4����������CeԪ�صĻ��ϼ۴ӣ�3�����ߵ���4�ۣ�ʧȥ1�����ӡ�˫��ˮ����Ԫ�صĻ��ϼ۴ӣ�1�۽��͵���2�ۣ�����ݵ��ӵ�ʧ�غ��ԭ���غ��֪��Ӧ�����ӷ���ʽΪ2Ce3++H2O2+6H2O��2Ce(OH)4��+6H+��

��ϰ��ϵ�д�
�����Ŀ
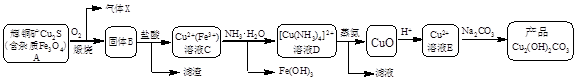
 Cu2+(aq) + 4NH3(aq)�������Ϲ������̣�����˵������ȷ����
Cu2+(aq) + 4NH3(aq)�������Ϲ������̣�����˵������ȷ���� CuO + 2HCl��+ 4NH3��
CuO + 2HCl��+ 4NH3��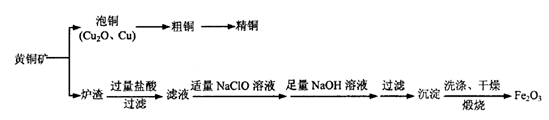
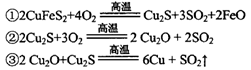
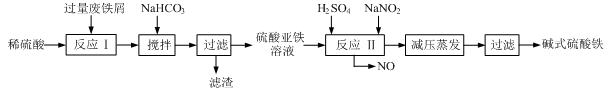
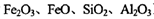 )���Ʊ�Fe2O3���������̻ش��������⣺
)���Ʊ�Fe2O3���������̻ش��������⣺