��Ŀ����
1����1.0L�ܱ������з���0.10molA��g������һ���¶Ƚ������·�Ӧ��A��g��?B��g��+C��g����H=+85.1kJ•mol-1����Ӧʱ�䣨t����������������ѹǿ��p�������ݼ��±���| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.91 | 5.58 | 6.32 | 7.31 | 8.54 | 9.50 | 9.52 | 9.53 | 9.53 |
��1�������A��ƽ��ת���ʣ�Ӧ��ȡ�Ĵ�ʩΪ�����¶ȡ�����ѹǿ��
��2������ѹǿp����ʼѹǿp0���㷴Ӧ��A��ת���ʦ���A���ı���ʽΪ$\frac{P-P{\;}_{0}}{P{\;}_{0}}$��100%��ƽ��ʱA��ת����Ϊ94.1%����ʽ�����㷴Ӧ��ƽ�ⳣ��K1.5mol/L��
��3��������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n�ܺͷ�Ӧ��A�����ʵ���n��A����n��=$\frac{0.10P}{P{\;}_{0}}$mol��n��A��=0.10����2-$\frac{p}{p{\;}_{0}}$��mol��
���±�Ϊ��Ӧ��AŨ���뷴Ӧʱ������ݣ�����a=0.051����
| ��Ӧʱ��t/h | 0 | 4 | 8 | 16 |
| c��A��/��mol•L-1�� | 0.10 | a | 0.026 | 0.0065 |
���� ��1����Ӧ�����ȷ�Ӧ����Ӧǰ��������������ƽ���ƶ�ԭ�������ж�ת���ʣ�
��2����ͬ������ѹǿ֮�ȵ������ʵ���֮�ȣ���Ӧǰ�����ʵ����������Ƿ�Ӧ��A�����ʵ��������ת���ʸ������õ������ݻ�ѧƽ������ʽ��ʽ����ƽ��Ũ�ȴﵽƽ�ⳣ����
��3����������ͬ������ѹǿ֮�ȵ������ʵ���֮�ȣ����ƽ�����õ���
������ƽ��A��Ũ�ȼ��㣬����ͼ�����ݷ����жϴ��ڵĹ��ɣ�
��� �⣺��1����һ���¶Ƚ������·�Ӧ��A��g��?B��g��+C��g ����H=+85.1kJ•mol-1����Ӧ�����ȷ�Ӧ����Ӧǰ���������������ƽ���ƶ�ԭ��������֪�������A��ƽ��ת���ʣ�ƽ��������У��������»��ѹ������ʹƽ��������У�
�ʴ𰸣������¶ȡ�����ѹǿ��
��2����Ӧǰ���������ʵ���������ڷ�Ӧ��A��������������ѹǿp����ʼѹǿp0���㷴Ӧ��A��ת���ʦ���A���ı���ʽ=$\frac{P-P{\;}_{0}}{P{\;}_{0}}$��100%��
ƽ��ʱA��ת����=$\frac{9.53-4.91}{4.91}$��100%=94.1%��
���ݻ�ѧƽ������ʽ ��ʽ�õ���
A��g��?B��g��+C��g ��
��ʼ����mol/L�� 0.10 0 0
�仯����mol/L�� 0.10��94.1% 0.10��94.1% 0.10��94.1%
ƽ������mol/L��0.10��1-94.1%�� 0.10��94.1% 0.10��94.1%
K=$\frac{c��B��c��C��}{c��A��}$=$\frac{��0.0941mol/L��{\;}^{2}}{0.10����1-94.1%��}$=1.5mol/L
�ʴ�Ϊ$\frac{P-P{\;}_{0}}{P{\;}_{0}}$��100%��94.1%�� 1.5mol/L��
��3��������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n���ͷ�Ӧ��A�����ʵ���n��A��������ѹǿ֮�ȵ������ʵ���֮�ȣ�n����n��ʼ=P��P0 ��n��=$\frac{0.10P}{P{\;}_{0}}$��
A��g��?B��g��+C��g ��
��ʼ����mol�� 0.10 0 0
�仯����mol�� x x x
ijʱ������mol�� 0.10-x x x
��0.10+x����0.10=P��P0
x=$\frac{0.10��P-P{\;}_{0}��}{P{\;}_{0}}$
n��A��=0.10-$\frac{0.10��P-P{\;}_{0}��}{P{\;}_{0}}$=0.10����2-$\frac{p}{p{\;}_{0}}$��mol��
�ʴ�Ϊ��$\frac{0.10P}{P{\;}_{0}}$��0.10����2-$\frac{p}{p{\;}_{0}}$����
��n��A��=0.10����2-$\frac{p}{p{\;}_{0}}$ ��=0.10����2-$\frac{7.31}{4.91}$ ��=0.051mol����Ũ��a=0.051mol/L����������������֪��ÿ��4h��A��Ũ�ȼ�Сһ�룬�ʴ�Ϊ��0.051���ﵽƽ��ǰÿ���4h��c��A������Լһ�룻�ɴ˹����Ƴ���Ӧ��12hʱ��Ӧ���Ũ��c��A��=$\frac{0.026mol/L}{2}$=0.013mol/L��
�ʴ�Ϊ��0.051��ÿ��4h��A��Ũ�ȼ�Сһ�룻0.013��
���� ���⿼��ѹǿ��ϵ�����ʵ����ļ���Ӧ�ã���ѧƽ����㷽����ͼ�����ݴ��������ķ����жϣ���Ŀ�Ѷ��еȣ�

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�| A�� | ���� | B�� | ���� | C�� | ������ | D�� | ��ԭ�� |
| A�� | NH4HCO3��Һ�м������NaOH��Һ��HCO3-+OH-�TH2O+CO2 | |
| B�� | �����������Һ��ͨ������������̼��2ClO-+H2O+CO2�T2HClO+CO32- | |
| C�� | ���Ҷ��ᣨH2C2O4����Һ�е�����������������Һ��H2C2O4+2OH-�TC2O42-+2H2O | |
| D�� | ���Ȼ�������Һ�м���ϡ���3Fe2++4H++NO3-�T3Fe3++NO��+2H2O |
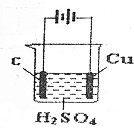
| A�� | ����һ������ | |
| B�� | C��������Cu������ | |
| C�� | ���Ĺ���ʵ�����ǵ��ˮ | |
| D�� | ʯī�缫�ϵķ�Ӧ��4OH--4e-=O2��+2H20 |
| ���� | X | Y | Z |
| ��ʼŨ��/��mol•L-1�� | 0.1 | 0.2 | 0 |
| ƽ��Ũ��/��mol•L-1�� | 0.05 | 0.05 | 0.1 |
| A�� | ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% | |
| B�� | �÷�Ӧ�Ļ�ѧ����ʽΪ3X��g��+Y��g��?2Z��g�� | |
| C�� | 25��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ1600 | |
| D�� | �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ�� |
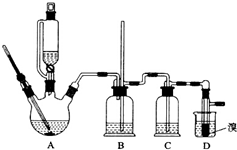 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH $��_{170��}^{H_{2}SO_{4}��Ũ��}$ CH2=CH2
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�
������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
��1���ڴ��Ƹ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d��������ȷѡ��ǰ����ĸ��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2����װ��C��Ӧ����c����Ŀ�������շ�Ӧ�п������ɵ��������壺������ȷѡ��ǰ����ĸ��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ��Ƹ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ����¡�����
��5����������������δ��Ӧ��Br2�������bϴ�ӳ�ȥ��������ȷѡ��ǰ����ĸ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��6�������������������������ѣ���������ķ�����ȥ��
��7����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
| �¶�/�� | 700 | 800 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| A�� | 4sʱc��B��Ϊ0.76mol/L | |
| B�� | 830���ƽ��ʱ��A��ת����Ϊ80% | |
| C�� | ��Ӧ��ƽ��������¶ȣ�ƽ�������ƶ� | |
| D�� | 1200��ʱ��ӦC��g��+D��g��?A��g��+B��g����ƽ�ⳣ����ֵΪ0.4 |
 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩 �ߵζ���
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩 �ߵζ���