��Ŀ����
9��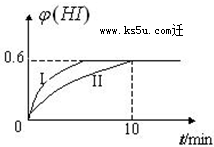 ��1mol I2��g�� ��2mol H2��g������2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0��2minʱ�����I2�����ʵ���Ϊ0.6mol��10min��ﵽƽ�⣬HI����������գ�H����ʱ��仯����ͼ������II��ʾ��
��1mol I2��g�� ��2mol H2��g������2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0��2minʱ�����I2�����ʵ���Ϊ0.6mol��10min��ﵽƽ�⣬HI����������գ�H����ʱ��仯����ͼ������II��ʾ����ش��������⣺
��1��ǰ2min�ڣ���H2��ʾ��ƽ����Ӧ����Ϊ0.1 mol•L-1•min-1��
��2���ﵽƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ0.05mol•L-1��
��3�����ı䷴Ӧ��������ij�����¦գ�HI���ı仯����ͼ����I��ʾ��������������Ǣۢݣ�����������������ţ�
�ٺ��������£������¶� �ں��������£������¶�
�ۺ��������£���С��Ӧ��������� �ܺ��������£��������������
�ݺ��º��������£������ʵ��Ĵ�����
���� ��1������2minʱ�����I2�����ʵ���Ϊ0.6mol���V=$\frac{��c}{��t}$�����ķ�Ӧ���ʣ��ٸ��ݷ�Ӧ����֮�ȵ���ϵ��֮�ȼ���H2��ƽ����Ӧ���ʣ�
��2������ԭ���غ���������ʵ���Ũ�ȣ�
��3���÷�Ӧ��һ����Ӧǰ�������������Ŀ��淴Ӧ���ı�����ʱ���÷�Ӧ��HI������������䣬˵���ı����ƽ�ⲻ�ƶ����ı�����ʱ����ƽ���ʱ�����̣�˵���ӿ췴Ӧ���ʣ��ı����������������ѹǿ�����������
��� �⣺��1��2minʱ�����I2�����ʵ���Ϊ0.6mol����V��I2��=V��H2��=$\frac{��c}{��t}$=$\frac{\frac{1-0.6}{2}}{2}$=0.1 mol•L-1•min-1���ʴ�Ϊ��0.1 mol•L-1•min-1��
��2����ij�ʼŨ��=$\frac{1mol}{2L}$=0.5mol/L���÷�Ӧǰ������������䣬ƽ��ʱ��HI���������Ϊ0.6����n��HI��=3mol��0.6=1.8mol��C��HI��=$\frac{1.8mol}{2L}$=0.9mol/L������Iԭ���غ��C��HI��+2C��I2��=0.5mol/L��2��C��I2��=$\frac{1-0.9}{2}$mol/L=0.05mol•L-1���ʴ�Ϊ��0.05��
��3���÷�Ӧ��һ����Ӧǰ�������������Ŀ��淴Ӧ���ı�����ʱ���÷�Ӧ��HI������������䣬˵���ı����ƽ�ⲻ�ƶ����ı�����ʱ����ƽ���ʱ�����̣�˵���ӿ췴Ӧ���ʣ��ı����������������ѹǿ�������������ѡ�ۢݣ�
���� ���⿼���˷�Ӧ���ʵļ����Լ����ʵ���Ũ�ȡ���������Ի�ѧ��Ӧƽ���ƶ���Ӱ���֪ʶ�㣬����ԭ���غ����ƽ��ʱ���Ũ�ȣ��ٽ��ͼ���з�Ӧʱ�䡢HI���������ȷ���ı�������ע�⣺���۷��ȷ�Ӧ�������ȷ�Ӧ���¶ȶ��ı仯ѧƽ���ƶ���Ϊ�״��㣻

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д���ͬѧ��ȡ����±����������NaOH��ˮ��Һ�����ȣ���ȴ����AgNO3��Һ�����е���ɫ�������ɣ���Ϊ�������
��ͬѧ��ȡ����±����������NaOH���Ҵ���Һ�����ȣ���ȴ���������ữ������AgNO3��Һ�����е���ɫ�������ɣ���Ϊ�������
���ڼס�����λͬѧ��ʵ��������ȷ���ǣ�������
| A�� | ��ͬѧ�ķ������� | |
| B�� | ��ͬѧ�ķ������� | |
| C�� | �ס�����λͬѧ�ķ������о����� | |
| D�� | �ס�����λͬѧ��ʵ�����漰��±����������һ�� |
| A�� | 78 gNa2O2�����������������Ӹ�����Ϊ2NA | |
| B�� | 1.5 g CH3+�к��еĵ�����ΪNA | |
| C�� | 3.4 g�����������0.6NA��N-H�� | |
| D�� | �����£�100 mL1 mol•L-1AlCl3��Һ��Al3+������������0.1NA |
| ��� | A | B | C | D |
ʵ�� װ�� |  | 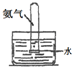 | 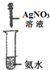 | 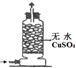 |
| ʵ�� Ŀ�� | ʵ����ģ������Ƽ�Ʊ�NH4HCO3 | ��֤NH3������ˮ | �Ʊ�������Һ | ����NH3 |
| A�� | 0.4mol | B�� | 0.3 mol | C�� | 0.2mol | D�� | 0.1 mol |
| A�� | 11��8 | B�� | 12��17 | C�� | 11��18 | D�� | 8��12 |
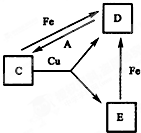 ��A��B��C��D��E��F���ֳ������ʣ������Ƿֱ�����ˮ��������Һ��ɫ������ͬ����֪��
��A��B��C��D��E��F���ֳ������ʣ������Ƿֱ�����ˮ��������Һ��ɫ������ͬ����֪��
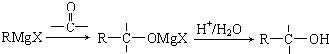
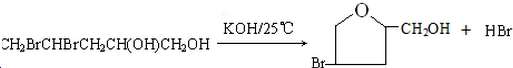 ����
����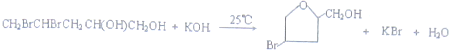 ������
������