��Ŀ����
19����֤��ijһ±����Ϊ��������ס�����ͬѧ��������·�������ͬѧ��ȡ����±����������NaOH��ˮ��Һ�����ȣ���ȴ����AgNO3��Һ�����е���ɫ�������ɣ���Ϊ�������
��ͬѧ��ȡ����±����������NaOH���Ҵ���Һ�����ȣ���ȴ���������ữ������AgNO3��Һ�����е���ɫ�������ɣ���Ϊ�������
���ڼס�����λͬѧ��ʵ��������ȷ���ǣ�������
| A�� | ��ͬѧ�ķ������� | |
| B�� | ��ͬѧ�ķ������� | |
| C�� | �ס�����λͬѧ�ķ������о����� | |
| D�� | �ס�����λͬѧ��ʵ�����漰��±����������һ�� |
���� ±��������������ˮ��Һ���������·���ˮ�ⷴӦ�����������ƴ���Һ���������·�����ȥ��Ӧ�������Ƿ�����Ԫ�أ��ڷ���ˮ�⡢��ȥ��Ӧ�����Һ��Ӧ�ȼ��������ữ����ֹ����AgOH������Ӱ��ʵ�������Դ˽����⣮
��� �⣺��±��������ȥ��Ӧ�����������㣺һ��������2��Cԭ�ӣ�����������±��ԭ�ӵ�Cԭ�����ڵ�Cԭ���ϱ�����Hԭ�ӣ��ڼ���±����������±�ص���ȷ������ȡ±������NaOH��Һ���ȣ���ȴ�����ϡ�����ữ���ټ�����������Һ���۲������ɫ��
���ַ���������������ͬѧ���ڼ��������ȴ��û�м���ϡ�����ữ����ͬѧ����������±��������ȥ��Ӧ����±�����е�±ԭ��ת��Ϊ±�����ӣ��ټ����������м��飬��±��������ȥ��Ӧ����ǰ��ģ���CH3Br�ȾͲ��ܷ�����ȥ��Ӧ�����Դ˷��о����ԣ�
��ѡC��
���� ���⿼�����ʵļ���ͼ����ʵ�鷽������ƺ����ۣ�Ϊ�߿��������ͺ�Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע��������ʵ������Լ�ʵ��������ԡ������Ե����ۣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
10����Ӧ2SO2+O2?2SO3���ܱ������н��У����й��ڸ÷�Ӧ��˵��������ǣ�������
| A�� | �����¶��ܼӿ췴Ӧ���� | B�� | ʹ��ǡ���Ĵ����ܼӿ췴Ӧ���� | ||
| C�� | ����O2��Ũ���ܼӿ췴Ӧ���� | D�� | SO2��O2��100%ת��ΪSO3 |
7���±���A��B��C��D�����л�����й���Ϣ��
��ش��������⣮
��1��д���л���C�Ľṹ��ʽHCOOH��
��2��д��B��D�Ļ�ѧ��Ӧ����ʽCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
��3��д��A��NH3��һ�������·�Ӧ���ɱ�ϩ�����Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�CH2=CHCOOH+NH3��CH2=CHCOONH2+H2O����Ӧ����ȡ����Ӧ��
��4����ϩ�����ж���ͬ���칹�壬��д��������ͬʱ����ȩ����̼̼˫����3��ͬ���칹��Ľṹ��ʽCH2=CHNHCHO��CH2=C��NH2��CHO��CH��NH2��=CHCHO��
| A | 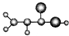 ����C��H��O����Ԫ����� �����ģ��Ϊ�� ������NH3��һ�������·�Ӧ���ɱ�ϩ����CH2=CHCONH2 ����Է�������Ϊ72 |
| B | ����C��H��O����Ԫ����ɡ�������Na��Ӧ����������NaOH��Һ��Ӧ ������A��Ӧ������Է�������Ϊ100���� |
| C | ����Է���������B��ͬ�����ܱ����Ƶ�������ͭ����Һ���� ������NaHCO3��Һ��Ӧ�ų�CO2���� |
| D | ����ʹ������Ȼ�̼��Һ��ɫ��������ˮ��һ�������·�Ӧ����B |
��1��д���л���C�Ľṹ��ʽHCOOH��
��2��д��B��D�Ļ�ѧ��Ӧ����ʽCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
��3��д��A��NH3��һ�������·�Ӧ���ɱ�ϩ�����Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�CH2=CHCOOH+NH3��CH2=CHCOONH2+H2O����Ӧ����ȡ����Ӧ��
��4����ϩ�����ж���ͬ���칹�壬��д��������ͬʱ����ȩ����̼̼˫����3��ͬ���칹��Ľṹ��ʽCH2=CHNHCHO��CH2=C��NH2��CHO��CH��NH2��=CHCHO��
14����v��������v���棩�ֱ��ʾ���淴Ӧ������Ӧ���ʺ��淴Ӧ���ʣ���һ���¶��¿��淴ӦN2+3H2$\frac{\underline{\;���¡���ѹ\;}}{����}$2NH3�ﵽƽ��ʱ��������
| A�� | V���棩��V������ | B�� | V���棩��V������ | ||
| C�� | V���棩��V�����������淴Ӧֹͣ | D�� | V���棩=V�����������淴Ӧ�Խ��� |
11��������Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ���ǣ�������
| A�� | 25��ʱpH=10��NaOH��Һ��pH=10�İ�ˮ�У�c��Na+����c��NH${\;}_{4}^{+}$�� | |
| B�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��CH3COOH�� | |
| C�� | ��NaHA��Һ�У�H2AΪ���ᣩ��c��Na+����c��HA-����c��OH-����c��H+�� | |
| D�� | �����£���0.01 mol•L-1 NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+����c��SO${\;}_{4}^{2-}$����c��NH${\;}_{4}^{+}$����c��OH-��=c��H+�� |
8��0.1mol NaHCO3���������
| A�� | 0.2mol Na+ | B�� | 0.05mol CO${\;}_{3}^{2-}$ | ||
| C�� | 6.02��1023��O | D�� | 0.1mol H |
 ��
��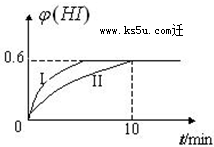 ��1mol I2��g�� ��2mol H2��g������2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0��2minʱ�����I2�����ʵ���Ϊ0.6mol��10min��ﵽƽ�⣬HI����������գ�H����ʱ��仯����ͼ������II��ʾ��
��1mol I2��g�� ��2mol H2��g������2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0��2minʱ�����I2�����ʵ���Ϊ0.6mol��10min��ﵽƽ�⣬HI����������գ�H����ʱ��仯����ͼ������II��ʾ��