��Ŀ����
����Ŀ����������һ�ָ�Ч�Ŀ�űҩ��ij�о���ѧϰС���ͬѧͨ���������ϻ��������Ϣ������������̼���⡢������Ԫ������ɵģ��۵�Ϊ156��157�棬�������������װ�������������صķ�����ɣ��ش��������⣺
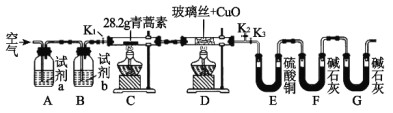
װ�� | ʵ��ǰ/g | ʵ���/g |
E | 22.6 | 42.4 |
F | 80.2 | 146.2 |
��1���Լ����������Ҫ���еIJ�����_____________����Ŀ����____________��ʵ������Ҫ�Կ���K1��K2��K3���в������״ζԿ���K1��K2��K3���в����ķ�����________���ʵ�ʱ����ٹر���Ӧ�Ŀ��أ�Ϊ���ʵ���ȷ�ԣ���C��ֹͣ���Ⱥ�Ӧ���еIJ�����____________��
��2���Լ�a��b�ֱ���________________________________________����ȼC��D���ƾ��Ƶ��Ⱥ�˳����___________________________��ʹ��װ��D��Ŀ����___________��
��3��E��ʹ������ͭ�����ʹ��CaCl2�ĺô�������������Ч���IJ����ԣ���_____��
��4����ַ�Ӧ���õ��й��������ϱ����������ɵ�����ȫ�������գ������������ص����ʽ��_________________________��
��5����Ҫ��������صķ���ʽ������Ҫͨ��ʵ�������һ�����ݣ�����ָ����������ʲô�������������ݵķ����ǣ�ֻ���������ϵĿ����ԣ���___________��
���𰸡���װ����ͨ�������ӵĿ��� �ų�װ���е�CO2��H2O(g)�Է�ֹ��������� ��K1��K2���ر�K3 ����ͨ����������� NaOH��Һ��Ũ���� �ȵ�ȼD���ľƾ��� ��C���������ɵ�CO��H2��ʱ����ΪCO2��H2O CO2��ȷ��ˮ�Ƿ�ȫ������ C15H22O5 ����ͬ��ͬѹ����������̬����������̬ˮ������������
��������
��ʵ��IJ���ԭ����ʹ���������ղ���CO2��ˮ��Ȼ����������ɵ�ˮ��CO2�������������������Ʒ��̼����Ԫ�ص������������ʵ�������һ�������Ԫ�ص����������ʵ��������������ʽ��
��1��ʵ�鿪ʼǰ����Ҫ�ų��������أ�װ���п��ܴ��ڵ�CO2��H2O(g)����ǰ��ȥ��������װ��ͨ�������ʵ�鿪ʼ����Ҫ��K1��K2���ر�K3��C��ֹͣ���Ⱥ������ͨ����������ӣ��Ա��ܽ����������ղ���CO2��ˮ��ȫ��������װ���У���֤ʵ���ȷ�ԡ�
��2��Ϊȷ��E��F�������յ�����������������յIJ��ʵ��ǰӦ�Ƚ������е�CO2��H2O(g)��ȥ�����Լ�a��bӦ�ֱ�Ϊ����Һ��Ũ���ͬʱΪȷ�������ص����ղ�����ȫ�������գ�ֹͣ���Ⱥ�����Ҫͨ�������Ӹ���Ŀ�����װ��D����;�ǽ�C�п������ɵ�CO��H2����ΪCO2��ˮ��ʵ��ʱӦ�ȵ�ȼD���ƾ�����ȷ��C�����ܲ�����CO��H2�ܱ���ʱ������
��3��������ˮ����ͭ��ˮ������ɫ���ʿ�ͨ���۲�U�ι��Ҳ�����Ƿ�ȫ������ɫ�ж�ˮ�Ƿ���ȫ���ա�
��4��![]() ��
��![]() ��
��![]() ��
��![]() ����������������ԭ�ӵ�����Ϊ
����������������ԭ�ӵ�����Ϊ![]() ��
��![]() ��
��![]() ���ʷ���ʽΪC15H22O5��
���ʷ���ʽΪC15H22O5��
��5�������ʽȷ���л���ķ���ʽ����Ҫ֪�������ص���Է�����������ͨ������ͬ��ͬѹ�µ���������̬����������̬ˮ�������Ȼ����������ȵ���Ħ�������ķ���ȷ����Ħ��������Ҳ��ֱ�Ӳ���һ��������������������һ���¶ȡ�ѹǿ�µ������Ȼ��������Է���������

 ����������������ϵ�д�
����������������ϵ�д�����Ŀ����������ĵ���ƽ�ⳣ�������
���� | HCOOH | HClO | H2CO3 | H2SO3 |
����ƽ�� ������25�棩 | Ka��1.77��10��4 | Ka��4.0��10��8 | Ka1��4.3��10��7 Ka2��4.7��10��11 | Ka1��1.54��10��2 Ka2��1.02��10��7 |
��1�������¢�0.1mol��L-1HCOONa����0.1mol��L-1NaClO����0.1mol��L-1Na2CO3����0.1mol��L-1NaHCO3������Һ��pH�ɴ�С�Ĺ�ϵΪ________________�����������գ�
��2��Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ________________��
��3���������ӷ���ʽ��ȷ����___________������ĸ����
A.2ClO-+H2O+CO2=2HClO+CO32- B.2HCOOH+CO32-=2HCOO-+H2O+CO2��
C.H2SO3+2HCOO-=2HCOOH+SO32- D.Cl2+H2O+2CO32-=2HCO3-+Cl-+ClO-
��4��ij�¶ȣ�T�棩�µ���Һ�У�c��H+��=10-xmol��L-1��c��OH-=10-ymol��L-1��x��y�Ĺ�ϵ��ͼ��ʾ��
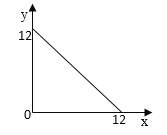
�ٴ��¶��£�0.01mol/L��NaOH��Һ��ˮ�������OH-Ũ��Ϊ_______��
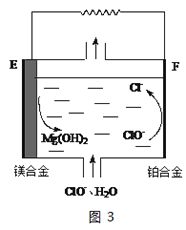
���ڴ��¶��£�0.1mol��L-1��NaHSO4��Һ��0.1mol��L-1��Ba(OH)2��Һ���±��мס��ҡ���������ͬ��ʽ��ϣ�
�� | �� | �� | �� | |
0.1mol��L-1��Ba��OH��2 | 10 | 10 | 10 | 10 |
0.1mol��L-1��NaHSO4 | 5 | 10 | 15 | 20 |
����ʽ��Ϻ�������Һ��pHΪ____________��
���ҷ�ʽ��Ϻ��䷴Ӧ�����ӷ���ʽ��_________________��
������ʽ��Ϻ�������Һ��____________����������������������������
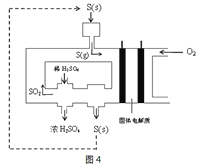
����Ŀ�����������һ�ֳ�������������Ҫ���ڻ�������������ҩ��ҵ�ȡ��ɽ����̿�(��Ҫ�ɷ�ΪMnO2)��KClO3�ڼ��Խ������Ƶ�K2MnO4��Ȼ��ͨ��CO2�Ʊ�������ء���֪��
�¶� | �ܽ��/g | ||||
K2CO3 | KHCO3 | KMnO4 | K2SO4 | CH3COOK | |
20�� | 111 | 33.7 | 6.38 | 11.1 | 217 |
(1)�Ʊ�����ص���Ҫ��ӦΪ��3MnO2+6KOH+KClO3=3K2MnO4+KCl+3H2O
���÷�Ӧ�е���������_____________����ԭ����_____________��
��ÿ���� 1mol K2MnO4ת��_______ mol ���ӡ�
(2)ͨ������CO2����ʱ������ط����绯��Ӧ������KMnO4��MnO2��K2CO3��
�������ɵ� KMnO4��MnO2�����ʵ���֮��Ϊ__________��
����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��ͣ���ԭ����______________________________������ͨCO2��Ϊ���������ᡣ�������Ϸ�����ѡ����������_______���õ��IJ�Ʒ���ȸ��ߡ�
A.���� B.Ũ���� C.ϡ����