��Ŀ����
����Ŀ����������ĵ���ƽ�ⳣ�������
���� | HCOOH | HClO | H2CO3 | H2SO3 |
����ƽ�� ������25�棩 | Ka��1.77��10��4 | Ka��4.0��10��8 | Ka1��4.3��10��7 Ka2��4.7��10��11 | Ka1��1.54��10��2 Ka2��1.02��10��7 |
��1�������¢�0.1mol��L-1HCOONa����0.1mol��L-1NaClO����0.1mol��L-1Na2CO3����0.1mol��L-1NaHCO3������Һ��pH�ɴ�С�Ĺ�ϵΪ________________�����������գ�
��2��Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ________________��
��3���������ӷ���ʽ��ȷ����___________������ĸ����
A.2ClO-+H2O+CO2=2HClO+CO32- B.2HCOOH+CO32-=2HCOO-+H2O+CO2��
C.H2SO3+2HCOO-=2HCOOH+SO32- D.Cl2+H2O+2CO32-=2HCO3-+Cl-+ClO-
��4��ij�¶ȣ�T�棩�µ���Һ�У�c��H+��=10-xmol��L-1��c��OH-=10-ymol��L-1��x��y�Ĺ�ϵ��ͼ��ʾ��
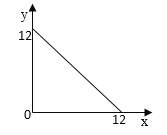
�ٴ��¶��£�0.01mol/L��NaOH��Һ��ˮ�������OH-Ũ��Ϊ_______��
���ڴ��¶��£�0.1mol��L-1��NaHSO4��Һ��0.1mol��L-1��Ba(OH)2��Һ���±��мס��ҡ���������ͬ��ʽ��ϣ�
�� | �� | �� | �� | |
0.1mol��L-1��Ba��OH��2 | 10 | 10 | 10 | 10 |
0.1mol��L-1��NaHSO4 | 5 | 10 | 15 | 20 |
����ʽ��Ϻ�������Һ��pHΪ____________��
���ҷ�ʽ��Ϻ��䷴Ӧ�����ӷ���ʽ��_________________��
������ʽ��Ϻ�������Һ��____________����������������������������
���𰸡��ۣ��ڣ��ܣ��� c��SO32-����c��CO32-����c��HCO3-����c��HSO3-�� BD 1��10-10mol��L-1 11 Ba2����OH����H����SO42��=BaSO4����H2O ��
��������
��1���ɵ��볣����֪����ͬ�����£���ĵ���ƽ�ⳣ��ԽС����ĵ���̶�ԽС��������ӵ�ˮ��̶�Խ����ͬŨ�ȵ�������ҺpHԽ��
��2��������Һ�е�ˮ��̶�Խ����Һ�������Ũ��ԽС��
��3���ɵ��볣���ж���ĵ���̶ȴ�С����ǿ���������ԭ���жϷ�Ӧ�Ƿ�����
��4��Kw=c��H+��c��OH-��=10-x10-y=10-��x+y��������ͼʾ��֪��x=12ʱ��c��OH-��=1mol/L����y=12ʱ��c��H+��=1mol/L����Kw=1��10-12��
��1���ɵ��볣����֪����ͬ�����£���ĵ���ƽ�ⳣ��ԽС����ĵ���̶�ԽС��������ӵ�ˮ��̶�Խ����ͬŨ�ȵ�������ҺpHԽ���ݵ���ƽ�ⳣ��֪���������ˮ��̶ȴ�С˳��ΪCO32-��ClO-��HCO3-��CH3COO-����������Һ��pH�ɴ�С�Ĺ�ϵΪ�����������������ʴ�Ϊ������������������
��2��������Һ�е�ˮ��̶�Խ����Һ�������Ũ��ԽС���ɵ��볣����֪��ˮ��̶�CO32-��SO32-����Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊc��SO32-����c��CO32-����c��HCO3-����c��HSO3-�����ʴ�Ϊ��c��SO32-����c��CO32-����c��HCO3-����c��HSO3-����
��3���ɵ��볣����֪��������ĵ���̶ȴ���̼���������ǿ���������ԭ����֪�������Դ���������̼�ᷴӦ���ɴ������̼�����ƣ���Ӧ�����ӷ���ʽΪClO-+H2O+CO2=HClO+HCO3-���ʴ���
B���ɵ��볣����֪������ĵ���̶ȴ���̼�ᣬ��ǿ���������ԭ����֪��������̼��������ᷴӦ���ɼ����ơ�������̼��ˮ����Ӧ�����ӷ���ʽΪ2HCOOH+CO32-=2HCOO-+H2O+CO2��������ȷ��
C���ɵ��볣����֪������ĵ���̶�С������������������������ǿ���������ԭ����֪���������������ᷴӦ�������������ƺͼ��ᣬ��Ӧ�����ӷ���ʽΪH2SO3+HCOO-=HCOOH+HSO3-���ʴ���
D���ɵ��볣����֪��������ĵ���̶�С��̼�����̼���������ǿ���������ԭ����֪����ˮ������ʹ�����������̼������Һ��Ӧ�����Ȼ��ơ�̼�����ƺʹ������ƣ���Ӧ�����ӷ���ʽΪCl2+H2O+2CO32-=2HCO3-+Cl-+ClO-������ȷ��
BD��ȷ���ʴ�Ϊ��BD��
��4��Kw=c��H+��c��OH-��=10-x10-y=10-��x+y��������ͼʾ��֪��x=12ʱ��c��OH-��=1mol/L����y=12ʱ��c��H+��=1mol/L����Kw=1��10-12��
�����¶��£�0.01mol/L��NaOH��Һ�У�c��OH-��=0.01mol/L��ˮ�������������Ũ�ȵ���ˮ�������OH-Ũ�ȣ���ˮ�������OH-Ũ��Ϊ![]() =1��10-10 mol/L���ʴ�Ϊ��1��10-10 mol/L��
=1��10-10 mol/L���ʴ�Ϊ��1��10-10 mol/L��
��0.1mol��L-1��NaHSO4��Һ��������Ũ��Ϊ0.1mol/L�� 0.1mol��L-1��Ba(OH)2��Һ������������Ũ��Ϊ0.2mol/L��
��������ݿ�֪������ʽ��Ϻ�Ba(OH)2��Һ��������Ӧ����Һ��OH-Ũ��Ϊ![]() =0.1mol/L������Һ��pHΪ11���ʴ�Ϊ��11��
=0.1mol/L������Һ��pHΪ11���ʴ�Ϊ��11��
��������ݿ�֪�����ҷ�ʽ��Ϻ�����������Һ����������������Һ��Ӧ�������ᱵ�������������ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2����OH����H����SO42��=BaSO4����H2O���ʴ�Ϊ��Ba2����OH����H����SO4/span>2��=BaSO4����H2O��
��������ݿ�֪��������ʽ��Ϻ������Ӻ�����������ǡ����ȫ��Ӧ����������Ӳ��ֳ���������Һ�����ԣ��ʴ�Ϊ�����ԡ�

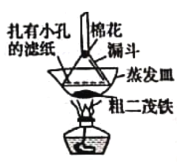
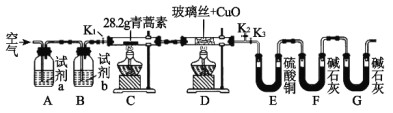
����Ŀ����������һ�ָ�Ч�Ŀ�űҩ��ij�о���ѧϰС���ͬѧͨ���������ϻ��������Ϣ������������̼���⡢������Ԫ������ɵģ��۵�Ϊ156��157�棬�������������װ�������������صķ�����ɣ��ش��������⣺
װ�� | ʵ��ǰ/g | ʵ���/g |
E | 22.6 | 42.4 |
F | 80.2 | 146.2 |
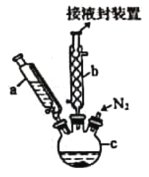
��1���Լ����������Ҫ���еIJ�����_____________����Ŀ����____________��ʵ������Ҫ�Կ���K1��K2��K3���в������״ζԿ���K1��K2��K3���в����ķ�����________���ʵ�ʱ����ٹر���Ӧ�Ŀ��أ�Ϊ���ʵ���ȷ�ԣ���C��ֹͣ���Ⱥ�Ӧ���еIJ�����____________��
��2���Լ�a��b�ֱ���________________________________________����ȼC��D���ƾ��Ƶ��Ⱥ�˳����___________________________��ʹ��װ��D��Ŀ����___________��
��3��E��ʹ������ͭ�����ʹ��CaCl2�ĺô�������������Ч���IJ����ԣ���_____��
��4����ַ�Ӧ���õ��й��������ϱ����������ɵ�����ȫ�������գ������������ص����ʽ��_________________________��
��5����Ҫ��������صķ���ʽ������Ҫͨ��ʵ�������һ�����ݣ�����ָ����������ʲô�������������ݵķ����ǣ�ֻ���������ϵĿ����ԣ���___________��