��Ŀ����
537�桢1.01��105Pa ʱ�����ݻ��ɱ���ܱ������г���2mol SO2�� 1mol O2����ʱ���������Ϊ200L���������м�����������壩�����ֺ��º�ѹ��������Ӧ��
2SO2������+O2������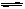 2SO3������
2SO3������
�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ0.91��
�Իش��������⣺
��1����ҵ�϶�������Ĵ��������ó�ѹ�������ø�ѹ��ԭ���ǣ� _______��
��2�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2mol SO3����������������ƽ��ʱ��SO2����������� �����������Ϊ L��
��3���¶��Ա���537�棬�����������200L���䣨���ݣ�������a molSO2��b molO2������������������Ӧ��ƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ1.01��105Pa����a:b=2:1����a= ��
2SO2������+O2������
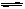 2SO3������
2SO3�������ﵽƽ��ʱ��ƽ��������SO3���������Ϊ0.91��
�Իش��������⣺
��1����ҵ�϶�������Ĵ��������ó�ѹ�������ø�ѹ��ԭ���ǣ� _______��
��2�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2mol SO3����������������ƽ��ʱ��SO2����������� �����������Ϊ L��
��3���¶��Ա���537�棬�����������200L���䣨���ݣ�������a molSO2��b molO2������������������Ӧ��ƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ1.01��105Pa����a:b=2:1����a= ��
��1����ѹ�£���������ĺ����Ѵﵽ91%���ӽ��ͳɱ����ǣ�û�б�Ҫ�ټ�ѹ��
��2 ��6%��137.5L��
��3��2.9��
����2molSO3������2molSO2��1molO2�൱��������������ͬʱ���Դﵽ��ͬ��ƽ��״̬������ƽ��ʱ������������������6%���������������Ϊ3%����ƽ��ʱSO3�����ʵ���Ϊxmol������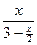 =0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=
=0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=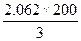 =137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ
=137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ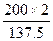 =2.9mol��
=2.9mol��
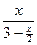 =0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=
=0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=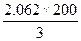 =137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ
=137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ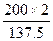 =2.9mol��
=2.9mol��
��ϰ��ϵ�д�
�����Ŀ
 3C(g)�ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ3mol��2mol��4mol�����¶Ȳ��䣬�������ڵ�ƽ���������ټ���A��C��lmol����ʱ��ƽ���ƶ��ķ���Ϊ
3C(g)�ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ3mol��2mol��4mol�����¶Ȳ��䣬�������ڵ�ƽ���������ټ���A��C��lmol����ʱ��ƽ���ƶ��ķ���Ϊ CO2��g��+H2��g������ʱ��
CO2��g��+H2��g������ʱ�� ��COת��ΪCO2������ƽ��״̬�»��������CO2�����������
��COת��ΪCO2������ƽ��״̬�»��������CO2����������� C(g)+D(g)�ں������Ѵ�ƽ��ı�־�ǣ� ��
C(g)+D(g)�ں������Ѵ�ƽ��ı�־�ǣ� �� N2O3+O2��N2O3�ְ�N2O3
N2O3+O2��N2O3�ְ�N2O3 2�ӣ������磩���������磩���ﵽƽ��״̬���ӣ����ķֽ���Ϊ40������ƽ�������ܶ�����ͬ�����£����ܶȵĶ��ٱ�?
2�ӣ������磩���������磩���ﵽƽ��״̬���ӣ����ķֽ���Ϊ40������ƽ�������ܶ�����ͬ�����£����ܶȵĶ��ٱ�? 2O3��ƽ����ϵ����֪O2��ת����Ϊ20%������Ũ��(c)�仯������ȷ����( )
2O3��ƽ����ϵ����֪O2��ת����Ϊ20%������Ũ��(c)�仯������ȷ����( )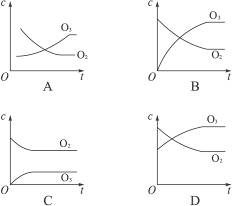
 2Z(g)���ﵽƽ�⣬��YŨ�ȸı��ʾ�ķ�Ӧ����v(��)��v(��)��ʱ��Ĺ�ϵ��ͼ����Y��ƽ��Ũ�ȱ���ʽ��ȷ���ǣ�ʽ��Sָ��Ӧ����������� ��
2Z(g)���ﵽƽ�⣬��YŨ�ȸı��ʾ�ķ�Ӧ����v(��)��v(��)��ʱ��Ĺ�ϵ��ͼ����Y��ƽ��Ũ�ȱ���ʽ��ȷ���ǣ�ʽ��Sָ��Ӧ����������� ��
