��Ŀ����
Ϊ��̽��������̬�����SO2��NO2��CO2�������ʣ�ijͬѧ�����һ��ʵ�飺
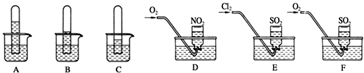
ʵ��һ��̽������������ˮ�е��ܽ��ԣ�����֧��ͬ���Թ��ռ����������壬������ʢ��ˮ���ձ��У�һ��ʱ��۲쵽��������ͼA��B��C��ʾ��
��1������ͬ�����£�����������ˮ���ܽ�������� ��д��A�ձ��з�����Ӧ�Ļ�ѧ����ʽ�� ���������ֻ�ձ��зֱ�μ�����ɫʯ����Һ���ɹ۲쵽�������� ��
ʵ���������ֻ����ƿ�ռ��������������������壬Ȼ���䵹����ˮ���У��ֱ���ͨ������O2��Cl2����ͼD��E��F��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�࣮
��2��ʵ�����װ��D�ļ���ƿ���ճ�����Һ������ƿ��Һ�岻��ɢ����
��д��װ��D���ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
�ڼ����ʵ������Ϊ��״�����ռ������壬��װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ ��
��3��ʵ��ǰ��Fװ�õ�ˮ����μӼ�����ɫʯ����Һ���۲쵽�������� ��ͨ����������Һ�����Խ� �����ǿ�����������������ǡ����䡱��д����Ӧ�Ļ�ѧ����ʽ�� ��
��4����Һ��������ƿ����Eװ�õ�ˮ����μ��Ȼ�����Һ�����ܹ۲쵽������Ϊ ��д���йط�Ӧ�����ӷ���ʽ ��
ʵ��һ��̽������������ˮ�е��ܽ��ԣ�����֧��ͬ���Թ��ռ����������壬������ʢ��ˮ���ձ��У�һ��ʱ��۲쵽��������ͼA��B��C��ʾ��
��1������ͬ�����£�����������ˮ���ܽ��������
ʵ���������ֻ����ƿ�ռ��������������������壬Ȼ���䵹����ˮ���У��ֱ���ͨ������O2��Cl2����ͼD��E��F��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�࣮
��2��ʵ�����װ��D�ļ���ƿ���ճ�����Һ������ƿ��Һ�岻��ɢ����
��д��װ��D���ܷ�Ӧ�Ļ�ѧ����ʽ��
�ڼ����ʵ������Ϊ��״�����ռ������壬��װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ
��3��ʵ��ǰ��Fװ�õ�ˮ����μӼ�����ɫʯ����Һ���۲쵽��������
��4����Һ��������ƿ����Eװ�õ�ˮ����μ��Ȼ�����Һ�����ܹ۲쵽������Ϊ

���㣺����ʵ�鷽�������,��������������ʼ���Ի�����Ӱ��,��������Ļ�ѧ����
ר�⣺ʵ����,Ԫ�ؼ��仯����
��������1��������������̼���ܽ�Ȳ�����������ˮ����������ԭ��Ӧ�����ܽ�����������������ˮ�õ�����Һ��Ϊ����Һ��
��2����D�ж���������ˮ��������Ӧ�������
��ˮ���������ƿ�����c=
���㣻
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ���ͨ������������2SO2+O2+2H2O=H2SO4��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl���ٵμ��Ȼ�����Һ���������ᱵ������
��2����D�ж���������ˮ��������Ӧ�������
��ˮ���������ƿ�����c=
| n |
| V |
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ���ͨ������������2SO2+O2+2H2O=H2SO4��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl���ٵμ��Ȼ�����Һ���������ᱵ������
���
�⣺��1��������������̼���ܽ�Ȳ�����������ˮ����������ԭ��Ӧ����ӦΪ3NO2+H2O�T2HNO3+NO�����ܽ�����������������ˮ�õ�����Һ��Ϊ����Һ���ֱ�μ�����ɫʯ����Һ���ɹ۲쵽��������Һ�����ɫ��
�ʴ�Ϊ��NO2����A����3NO2+H2O�T2HNO3+NO����Һ�����ɫ��
��2����D�ж���������ˮ��������Ӧ�������ᣬ��ӦΪ4NO2+O2+2H2O�T4HNO3���ʴ�Ϊ��4NO2+O2+2H2O�T4HNO3��
��ˮ���������ƿ���輯��ƿ���ΪVL�������Һ�����ΪVL����4NO2+O2+2H2O�T4HNO3֪��n��NO2��=n��HNO3������������Һ���ʵ����ʵ���Ũ��Ϊc=
=
=0.045mol/L���ʴ�Ϊ��0.045mol/L��
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ��μӼ�����ɫʯ����Һ���۲쵽����������ɫ��Һ��죬��ͨ������������2SO2+O2+2H2O=2H2SO4��������ǿ��
�ʴ�Ϊ����ɫ��Һ��죻��ǿ��2SO2+O2+2H2O=2H2SO4��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl�����ӷ�ӦΪSO2+Cl2+2H2O=4H++SO42-+2Cl-���ٵμ��Ȼ�����Һ���۲쵽�������ᱵ��ɫ���������ӷ�ӦΪSO42-+Ba2+=BaSO4�����ʴ�Ϊ����ɫ������SO2+Cl2+2H2O=4H++SO42-+2Cl-��SO42-+Ba2+=BaSO4����
�ʴ�Ϊ��NO2����A����3NO2+H2O�T2HNO3+NO����Һ�����ɫ��
��2����D�ж���������ˮ��������Ӧ�������ᣬ��ӦΪ4NO2+O2+2H2O�T4HNO3���ʴ�Ϊ��4NO2+O2+2H2O�T4HNO3��
��ˮ���������ƿ���輯��ƿ���ΪVL�������Һ�����ΪVL����4NO2+O2+2H2O�T4HNO3֪��n��NO2��=n��HNO3������������Һ���ʵ����ʵ���Ũ��Ϊc=
| n |
| V |
| ||
| VL |
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ��μӼ�����ɫʯ����Һ���۲쵽����������ɫ��Һ��죬��ͨ������������2SO2+O2+2H2O=2H2SO4��������ǿ��
�ʴ�Ϊ����ɫ��Һ��죻��ǿ��2SO2+O2+2H2O=2H2SO4��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl�����ӷ�ӦΪSO2+Cl2+2H2O=4H++SO42-+2Cl-���ٵμ��Ȼ�����Һ���۲쵽�������ᱵ��ɫ���������ӷ�ӦΪSO42-+Ba2+=BaSO4�����ʴ�Ϊ����ɫ������SO2+Cl2+2H2O=4H++SO42-+2Cl-��SO42-+Ba2+=BaSO4����
���������⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬�������ʵ����ʼ�ʵ������з����ķ�ӦΪ���Ĺؼ������ط���������ʵ���������ۺϿ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����Һ����ˮ���������c��OH-��=1��10-13mol/L���������������Һ��һ�����Դ���������������ǣ�������
| A��Al3+��Na+��NO3-��Cl- |
| B��K+��Na+��Cl-��SO42- |
| C��K+��Na+��Cl-��CO32- |
| D��K+��I-��NO3-��Cl- |
���ⶨC3H7OH��C6H12��ɵĻ������̼����������Ϊ78%����˻�������������������ǣ�������
| A��8% | B��10% |
| C��22% | D��18% |
�����йط�Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H��298K��=-905kJ?mol-1��������ȷ���ǣ�������
| A������������������ڷ�Ӧ��������� |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�Ӧ��ÿ4 mol NH3��g��������������905 kJ���� |
| D���÷�ӦΪ���ȷ�Ӧ |
������ˮ����ȡ���д��������Һ����Ҫ�����Һ��HClO���ʵ���Ũ�ȣ���Ҫ������Һ��HClŨ�ȣ����д�ʩ���Բ��õ��ǣ�������
| A�����Ȼӷ�HCl |
| B����ˮʹƽ��������Ӧ�����ƶ� |
| C����NaOH�к�HCl |
| D����CaCO3�к�HCl |
 2012���Ϧ�����Ǵ�ѧ�о���Աչʾ��һ�������������壬���ܱ���ӡ����Ϳ�������������ϣ��Ƴɶ������������ĵ����豸����ش��������⣺
2012���Ϧ�����Ǵ�ѧ�о���Աչʾ��һ�������������壬���ܱ���ӡ����Ϳ�������������ϣ��Ƴɶ������������ĵ����豸����ش��������⣺