��Ŀ����
��ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺

(1)��֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��K��
������Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��֪��һ���¶��£���ͬһƽ����ϵ�и���Ӧ��ƽ�ⳣ�����£�
C(s)+CO2(g)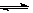 2CO(g)��K1
2CO(g)��K1
CO(g)+H2O(g)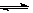 H2(g)+CO2(g)��K2
H2(g)+CO2(g)��K2
C(s)+H2O(g)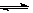 CO(g)+H2(g)��K3
CO(g)+H2(g)��K3
��K1��K2��K3֮��Ĺ�ϵΪ ��
(2)ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���������·�Ӧ��CO(g)+H2O(g) H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
�÷�Ӧ���淴Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£���Ӧ�ﵽƽ��ʱCO��ת����Ϊ ��
(3)���ڷ�ӦN2O4(g) 2NO2(g)����H=Q��Q>0�������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ�������� ������
2NO2(g)����H=Q��Q>0�������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ�������� ������
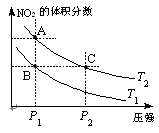
A�����ֲ�ͬ���¶ȱȽϣ�T1 > T2
B��A��C����ķ�Ӧ���ʣ�A<C
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���ʵ��
(4)����ͼ������NH3����ԭ����������һ��������ͨ�����ֲ�ͬ�����������ݳ������е������ﺬ�����Ӷ�ȷ�������ѵ��ʣ���Ӧԭ��Ϊ��NO(g) + NO2(g) + 2NH3(g) 2N2(g) + 3H2O(g)
2N2(g) + 3H2O(g)
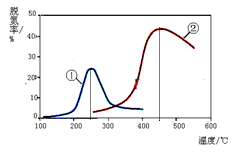
 ����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ�
����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ�
A��������Ӧ������ӦΪ���ȷ�Ӧ
B����ͬ�����£��ı�ѹǿ���ѵ���û��Ӱ��
C�����ߢ١�����ߵ��ʾ��ʱƽ��ת�������
D�������١��ڷֱ��ʺ���250���450�������ѵ�

(1)��֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��K��

������Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��֪��һ���¶��£���ͬһƽ����ϵ�и���Ӧ��ƽ�ⳣ�����£�
C(s)+CO2(g)
 2CO(g)��K1
2CO(g)��K1CO(g)+H2O(g)
 H2(g)+CO2(g)��K2
H2(g)+CO2(g)��K2C(s)+H2O(g)
 CO(g)+H2(g)��K3
CO(g)+H2(g)��K3��K1��K2��K3֮��Ĺ�ϵΪ ��
(2)ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���������·�Ӧ��CO(g)+H2O(g)
 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
(3)���ڷ�ӦN2O4(g)
 2NO2(g)����H=Q��Q>0�������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ�������� ������
2NO2(g)����H=Q��Q>0�������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ�������� ������
A�����ֲ�ͬ���¶ȱȽϣ�T1 > T2
B��A��C����ķ�Ӧ���ʣ�A<C
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���ʵ��
(4)����ͼ������NH3����ԭ����������һ��������ͨ�����ֲ�ͬ�����������ݳ������е������ﺬ�����Ӷ�ȷ�������ѵ��ʣ���Ӧԭ��Ϊ��NO(g) + NO2(g) + 2NH3(g)
 2N2(g) + 3H2O(g)
2N2(g) + 3H2O(g)
 ����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ�
����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ�A��������Ӧ������ӦΪ���ȷ�Ӧ
B����ͬ�����£��ı�ѹǿ���ѵ���û��Ӱ��
C�����ߢ١�����ߵ��ʾ��ʱƽ��ת�������
D�������١��ڷֱ��ʺ���250���450�������ѵ�
(1)��2�֣�C��s��+H2O��g�� CO��g��+H2��g�� K3=K1?K2
CO��g��+H2��g�� K3=K1?K2
(2)��2�֣����ȣ�75% ����1�֣�
(3)��2�֣�B��D ��һ��1�֣���һ����2�֣�
(4)��2�֣�D
 CO��g��+H2��g�� K3=K1?K2
CO��g��+H2��g�� K3=K1?K2(2)��2�֣����ȣ�75% ����1�֣�
(3)��2�֣�B��D ��һ��1�֣���һ����2�֣�
(4)��2�֣�D
��1��ƽ�ⳣ���Ķ���Ϊ�����淴Ӧ�ﵽƽ���������Ũ����֮���뷴Ӧ��Ũ����֮���ı�ֵ�����ԣ���ƽ�ⳣ������ʽK�� ��֪����Ӧ�ķ���ʽΪ��C��s��+H2O��g��
��֪����Ӧ�ķ���ʽΪ��C��s��+H2O��g�� CO��g��+H2��g����
CO��g��+H2��g����
�ɷ���ʽC(s)+CO2(g)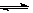 2CO(g)��K1�٣�CO(g)+H2O(g)
2CO(g)��K1�٣�CO(g)+H2O(g) 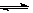 H2(g)+CO2(g)��K2�ڣ�
H2(g)+CO2(g)��K2�ڣ�
C(s)+H2O(g)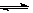 CO(g)+H2(g)��K3�����ۣ��ý���+��=�ۣ�����֪K1��K2��K3֮��Ĺ�ϵΪK3=K1?K2��
CO(g)+H2(g)��K3�����ۣ��ý���+��=�ۣ�����֪K1��K2��K3֮��Ĺ�ϵΪK3=K1?K2��
��2���ɱ���ƽ�ⳣ�����¶ȵĹ�ϵ���¶�Խ��KԽС������֪�淴Ӧ����ķ�ӦΪ���ȷ�Ӧ��
�ɷ���ʽ��CO(g) + H2O(g) H2(g) + CO2(g)��
H2(g) + CO2(g)��
��ʼŨ�ȣ�0.020mol/L 0.020mol/L 0 0
�仯Ũ�ȣ� x x x x
ƽ��Ũ�ȣ�0.020-x 0.020-x x x
500��ʱƽ�ⳣ��K=9=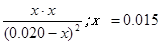 ��ƽ��ʱCO��ת����
��ƽ��ʱCO��ת����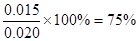
 ��
��
��3���ɷ���ʽN2O4(g) 2NO2(g)����H=Q��Q>0�����¶�Խ�ߣ�NO2(g)�ĺ���Խ�ߣ���T2 > T1��A������ͬ�¶��£�ѹǿԽ�����ԽС�������Ũ��Խ�ߣ���Ӧ����Խ�죬����A��C����ķ�Ӧ���ʣ�A<C��B��ȷ��B��C����NO2(g)�����������ͬ����B��C����������ƽ����Է���������B=C��C����B��A�Ĺ�����NO2(g)�ĵ�������������ü��ȵķ���ʵ�֣�D��ȷ��
2NO2(g)����H=Q��Q>0�����¶�Խ�ߣ�NO2(g)�ĺ���Խ�ߣ���T2 > T1��A������ͬ�¶��£�ѹǿԽ�����ԽС�������Ũ��Խ�ߣ���Ӧ����Խ�죬����A��C����ķ�Ӧ���ʣ�A<C��B��ȷ��B��C����NO2(g)�����������ͬ����B��C����������ƽ����Է���������B=C��C����B��A�Ĺ�����NO2(g)�ĵ�������������ü��ȵķ���ʵ�֣�D��ȷ��
��4�����ڷ�ӦΪ��NO(g) + NO2(g) + 2NH3(g) 2N2(g) + 3H2O(g)����ͼ���֪����ͬ�����£���ʼʱ���¶ȵ������ѵ������������¶ȵ������ѵ�����С����ߵ��ʾ�ﵽƽ��״̬��ƽ����������¶��ѵ��ʷ����Ǽ�С�ģ����ԣ��÷�Ӧ������Ӧ����Ϊ���ȷ�Ӧ��A�����÷�ӦΪ�ǵ������Ӧ��ѹǿ�ĸı�����Ӱ���ѵ��ʣ�����B�������ߢ١�����ߵ��ʾ��Ӧ�ﵽƽ��״̬��C������ͼ�η�����֪�����١��ڷֱ��ʺ���250���450�������ѵ���D��ȷ��
2N2(g) + 3H2O(g)����ͼ���֪����ͬ�����£���ʼʱ���¶ȵ������ѵ������������¶ȵ������ѵ�����С����ߵ��ʾ�ﵽƽ��״̬��ƽ����������¶��ѵ��ʷ����Ǽ�С�ģ����ԣ��÷�Ӧ������Ӧ����Ϊ���ȷ�Ӧ��A�����÷�ӦΪ�ǵ������Ӧ��ѹǿ�ĸı�����Ӱ���ѵ��ʣ�����B�������ߢ١�����ߵ��ʾ��Ӧ�ﵽƽ��״̬��C������ͼ�η�����֪�����١��ڷֱ��ʺ���250���450�������ѵ���D��ȷ��
 ��֪����Ӧ�ķ���ʽΪ��C��s��+H2O��g��
��֪����Ӧ�ķ���ʽΪ��C��s��+H2O��g�� CO��g��+H2��g����
CO��g��+H2��g�����ɷ���ʽC(s)+CO2(g)
 2CO(g)��K1�٣�CO(g)+H2O(g)
2CO(g)��K1�٣�CO(g)+H2O(g)  H2(g)+CO2(g)��K2�ڣ�
H2(g)+CO2(g)��K2�ڣ� C(s)+H2O(g)
 CO(g)+H2(g)��K3�����ۣ��ý���+��=�ۣ�����֪K1��K2��K3֮��Ĺ�ϵΪK3=K1?K2��
CO(g)+H2(g)��K3�����ۣ��ý���+��=�ۣ�����֪K1��K2��K3֮��Ĺ�ϵΪK3=K1?K2����2���ɱ���ƽ�ⳣ�����¶ȵĹ�ϵ���¶�Խ��KԽС������֪�淴Ӧ����ķ�ӦΪ���ȷ�Ӧ��
�ɷ���ʽ��CO(g) + H2O(g)
 H2(g) + CO2(g)��
H2(g) + CO2(g)����ʼŨ�ȣ�0.020mol/L 0.020mol/L 0 0
�仯Ũ�ȣ� x x x x
ƽ��Ũ�ȣ�0.020-x 0.020-x x x
500��ʱƽ�ⳣ��K=9=
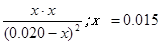 ��ƽ��ʱCO��ת����
��ƽ��ʱCO��ת����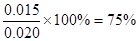
 ��
����3���ɷ���ʽN2O4(g)
 2NO2(g)����H=Q��Q>0�����¶�Խ�ߣ�NO2(g)�ĺ���Խ�ߣ���T2 > T1��A������ͬ�¶��£�ѹǿԽ�����ԽС�������Ũ��Խ�ߣ���Ӧ����Խ�죬����A��C����ķ�Ӧ���ʣ�A<C��B��ȷ��B��C����NO2(g)�����������ͬ����B��C����������ƽ����Է���������B=C��C����B��A�Ĺ�����NO2(g)�ĵ�������������ü��ȵķ���ʵ�֣�D��ȷ��
2NO2(g)����H=Q��Q>0�����¶�Խ�ߣ�NO2(g)�ĺ���Խ�ߣ���T2 > T1��A������ͬ�¶��£�ѹǿԽ�����ԽС�������Ũ��Խ�ߣ���Ӧ����Խ�죬����A��C����ķ�Ӧ���ʣ�A<C��B��ȷ��B��C����NO2(g)�����������ͬ����B��C����������ƽ����Է���������B=C��C����B��A�Ĺ�����NO2(g)�ĵ�������������ü��ȵķ���ʵ�֣�D��ȷ����4�����ڷ�ӦΪ��NO(g) + NO2(g) + 2NH3(g)
 2N2(g) + 3H2O(g)����ͼ���֪����ͬ�����£���ʼʱ���¶ȵ������ѵ������������¶ȵ������ѵ�����С����ߵ��ʾ�ﵽƽ��״̬��ƽ����������¶��ѵ��ʷ����Ǽ�С�ģ����ԣ��÷�Ӧ������Ӧ����Ϊ���ȷ�Ӧ��A�����÷�ӦΪ�ǵ������Ӧ��ѹǿ�ĸı�����Ӱ���ѵ��ʣ�����B�������ߢ١�����ߵ��ʾ��Ӧ�ﵽƽ��״̬��C������ͼ�η�����֪�����١��ڷֱ��ʺ���250���450�������ѵ���D��ȷ��
2N2(g) + 3H2O(g)����ͼ���֪����ͬ�����£���ʼʱ���¶ȵ������ѵ������������¶ȵ������ѵ�����С����ߵ��ʾ�ﵽƽ��״̬��ƽ����������¶��ѵ��ʷ����Ǽ�С�ģ����ԣ��÷�Ӧ������Ӧ����Ϊ���ȷ�Ӧ��A�����÷�ӦΪ�ǵ������Ӧ��ѹǿ�ĸı�����Ӱ���ѵ��ʣ�����B�������ߢ١�����ߵ��ʾ��Ӧ�ﵽƽ��״̬��C������ͼ�η�����֪�����١��ڷֱ��ʺ���250���450�������ѵ���D��ȷ��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CO2��g��+H2��g�� ��H��0
CO2��g��+H2��g�� ��H��0
 C B. 3A+2B
C B. 3A+2B B��D�����ֲ�ͬ�����½��У�B��D��ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���
B��D�����ֲ�ͬ�����½��У�B��D��ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±��� CH3CH2OH(g)+3H2O(g)��
CH3CH2OH(g)+3H2O(g)��  CO2ת����(%)
CO2ת����(%)
 2SO3����ƽ�ⳣ��K=19���ڸ��¶��µ����
2SO3����ƽ�ⳣ��K=19���ڸ��¶��µ���� �̶����ܱ������г�
�̶����ܱ������г�
 2
2










 ����HX����ԭ�ӽṹ����ԭ�� ��
����HX����ԭ�ӽṹ����ԭ�� �� ��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ�������������
��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ������������� ��Ӧ�ľ��ҳ̶�����
��Ӧ�ľ��ҳ̶�����