��Ŀ����
���¶�t1��t2�£�X2(g)�� H2��Ӧ����HX��ƽ�ⳣ�����±���
��1����֪t2��t1,HX�����ɷ�Ӧ�� ��Ӧ������ȡ����ȡ�����
��2��HX�ĵ���ʽ�� ��
��3�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳���� ��
��4��X2������H2��Ӧ ����HX����ԭ�ӽṹ����ԭ�� ��
����HX����ԭ�ӽṹ����ԭ�� ��
(5)K�ı仯���ֳ� ��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ�������������
��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ�������������
��6��������K�ı仯�������ƶϳ�������±��ԭ�Ӻ˵���������ӣ�_______��ѡ����ĸ��
a. ����ͬ�����£�ƽ���� ��ת��������
��ת��������
b. ��
�� ��Ӧ�ľ��ҳ̶�����
��Ӧ�ľ��ҳ̶�����
c. HX�Ļ�ԭ����
d. HX���ȶ�������
| ��ѧ����ʽ | K (t1 ) | K (t2) |
  2 2 | 1.8  |  |
   |  |  |
   |  |  |
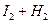  2HI 2HI | 43 | 34 |
��2��HX�ĵ���ʽ�� ��
��3�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳���� ��
��4��X2������H2��Ӧ
 ����HX����ԭ�ӽṹ����ԭ�� ��
����HX����ԭ�ӽṹ����ԭ�� ��(5)K�ı仯���ֳ�
 ��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ�������������
��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��__________��ԭ�Ӱ뾶�����õ���������������6��������K�ı仯�������ƶϳ�������±��ԭ�Ӻ˵���������ӣ�_______��ѡ����ĸ��
a. ����ͬ�����£�ƽ����
 ��ת��������
��ת��������b.
 ��
�� ��Ӧ�ľ��ҳ̶�����
��Ӧ�ľ��ҳ̶�����c. HX�Ļ�ԭ����
d. HX���ȶ�������

��

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ

 N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬
N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬

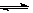 2CO(g)��K1
2CO(g)��K1 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
 2N2(g) + 3H2O(g)
2N2(g) + 3H2O(g)
 ����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ�
����˵����ȷ���ǣ�ע���ѵ��ʼ���������ת���ʣ� 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L����������·�Ӧ��ƽ�ⳣ��K= ��SO2��ƽ��ת����= ��
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L����������·�Ӧ��ƽ�ⳣ��K= ��SO2��ƽ��ת����= �� FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
 O�⣬����H
O�⣬����H �⣬����C
�⣬����C �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��



 = ��ֻ�����ֱ���ʽ��
= ��ֻ�����ֱ���ʽ�� ��ͬ
��ͬ ����
���� ��=����
��=���� xC��g������H��0��ƽ��ʱM��A��B��C�����ʵ���֮��Ϊ1��3��4�������ж���ȷ���� �� ��
xC��g������H��0��ƽ��ʱM��A��B��C�����ʵ���֮��Ϊ1��3��4�������ж���ȷ���� �� ��
