��Ŀ����
1������˵������ȷ���У��������ٺ��й��ۼ��Ļ����ﲻһ���ǹ��ۻ�����
������Ԫ���γɵ������Ӳ�һ��������8�����ȶ��ṹ
�۷ǽ���Ԫ���γɵĻ������еĻ�ѧ��һ���ǹ��ۼ�
��Ԫ�����ڱ�IA���ڵ�Ԫ��֮�䲻�����γ����Ӽ�
��NaCl����ˮ�ƻ������Ӽ����Ҵ�����ˮ�ƻ��˹��ۼ�
��Ԫ�����ڱ���һ�����ڵ�Ԫ��֮�䲻�����γ����Ӽ���
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
���� �ٺ��й��ۼ��Ļ����ﲻһ���ǹ��ۻ����
������Ԫ���γɵ������Ӳ�һ��������8�����ȶ��ṹ��
���Ȼ����笠������������������Ӽ������Էǽ���Ԫ���γɵĻ������еĻ�ѧ����һ���ǹ��ۼ���
��NaH�����ӻ����Ԫ�����ڱ�IA���ڵ�Ԫ��֮������γ����Ӽ���
��NaCl����ˮ�ƻ������Ӽ������Ҵ�����ˮδ�ƻ��˹��ۼ���
��Ԫ�����ڱ���һ�����ڵ�Ԫ�������뺤֮�䲻�����γ����Ӽ���
��� �⣺�ٺ��й��ۼ��Ļ����ﲻһ���ǹ��ۻ������NaOH������ȷ��
������Ԫ���γɵ������Ӳ�һ��������8�����ȶ��ṹ����H-������2���ӵ��ȶ��ṹ������ȷ��
�۷ǽ���Ԫ���γɵĻ������еĻ�ѧ����һ���ǹ��ۼ������Ȼ����笠������������������Ӽ����ʴ���
��Ԫ�����ڱ�IA���ڵ�Ԫ��֮������γ����Ӽ�����NaH�����ӻ�����ʴ���
��NaCl����ˮ�ƻ������Ӽ������Ҵ�����ˮδ�ƻ��˹��ۼ����ʴ���
��Ԫ�����ڱ���һ�����ڵ�Ԫ�������뺤֮�䲻�����γ����Ӽ�������ȷ��
��ѡB��
���� ������Ҫ�����˻�ѧ����ʵ�ʡ��γɡ���������Ҫ�����˻�ѧ���ڻ������еĴ��ڣ�

��ϰ��ϵ�д�
�����Ŀ
18�����в��������ʵ��ǣ�������
| A�� | ������Ӧ����ˮԡ���� | |
| B�� | ��ͨ��ʢ��ˮ��ϴ��ƿ�ķ�����ȥ���������к��е���ϩ���� | |
| C�� | ����ȩ��ԭ����Cu��OH��2����Һ��ʵ���У���Cu��OH��2����ҺʱӦ����NaOH��������ֱ�Ӽ��� | |
| D�� | ����������Һʱ����AgNO3��Һ���백ˮ�� |
19������˵����ȷ���ǣ�������
| A�� | ��${\;}_{1}^{2}$H��${\;}_{8}^{18}$O����ɵ�11gˮ������������Ϊ6NA | |
| B�� | H2O��D2O����Ϊͬ�������壬�����ߵĻ�ѧ�������� | |
| C�� | ${\;}_{8}^{18}$O2��${\;}_{8}^{16}$O3����Ϊͬλ�� | |
| D�� | ���ʯ��ʯī��Ϊͬ�������壬����֮���ת�����������仯 |
16������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ��뼸��AgNO3��Һ���г���������
��2���ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.03mol���������ɣ�ͬʱ�õ���Һ��
��3���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g
��4�������ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��
����˵������ȷ���ǣ�������
��1����һ�ݼ��뼸��AgNO3��Һ���г���������
��2���ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.03mol���������ɣ�ͬʱ�õ���Һ��
��3���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g
��4�������ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��
����˵������ȷ���ǣ�������
| A�� | ���裨3�������ȷ��CO32-һ�������� | |
| B�� | ���Բ��裨1����ʵ����۲���Ӱ�� | |
| C�� | ����ȷ��ԭ��Һ�Ƿ���K+��Cl- | |
| D�� | ���Ѳ��裨2������������ͨ�벽�裨1������Һ�У��ֿɲ���0.78g���� |
3���������У���Ҫ�Ի�ѧ��Ӧ�����ʺͻ�ѧ��Ӧ���Ƚ����о����Ա���ƻ�ѧ��Ӧ��
I��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ��������¶ȶԷ�Ӧ���ʵ�Ӱ�죮�������·������ʵ�飮
ͨ��ʵ��õ������������ʱ��Ĺ�ϵ��ͼ1��ʾ���ش��������⣺
��1������ʵ��ٵ�ͼʾ�Ǣ���
��2���Ա�ʵ��ۺܵ͢�Ŀ����̽�������Է�Ӧ���ʵ�Ӱ�죮
��3��ͨ������Ա�ʵ�飬���õ�ʵ������������������䣬����Ӧ��Ũ�Ȼ������¶Ȼ�����������ѧ��Ӧ���ʼӿ죮

��һ���¶��£������Ϊ2L�ĺ����ܱ������г���1molN2��3mol H2��һ�������·�����Ӧ��
N2��g��+3H2��g��?2NH3��g�����������N2���ʵ�����ʱ��仯��ͼ2��ʾ���ش��������⣺
��4���ӿ�ʼ��Ӧ��t2ʱ�̣�������ƽ����Ӧ����Ϊ$\frac{1}{2{t}_{2}}$mol/��L•min����
��5����t3ʱ�̣�������ת����Ϊ75%��
I��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ��������¶ȶԷ�Ӧ���ʵ�Ӱ�죮�������·������ʵ�飮
| ��Ӧ�� | ���� | �¶� | |
| �� | 10mL10% H2O2��Һ | �� | 25�� |
| �� | 10mL20% H2O2��Һ | �� | 25�� |
| �� | 10mL20% H2O2��Һ | �� | 40�� |
| �� | 10mL20% H2O2��Һ | 1��2��0.1mol/LFeCl3��Һ | 40�� |
��1������ʵ��ٵ�ͼʾ�Ǣ���
��2���Ա�ʵ��ۺܵ͢�Ŀ����̽�������Է�Ӧ���ʵ�Ӱ�죮
��3��ͨ������Ա�ʵ�飬���õ�ʵ������������������䣬����Ӧ��Ũ�Ȼ������¶Ȼ�����������ѧ��Ӧ���ʼӿ죮

��һ���¶��£������Ϊ2L�ĺ����ܱ������г���1molN2��3mol H2��һ�������·�����Ӧ��
N2��g��+3H2��g��?2NH3��g�����������N2���ʵ�����ʱ��仯��ͼ2��ʾ���ش��������⣺
��4���ӿ�ʼ��Ӧ��t2ʱ�̣�������ƽ����Ӧ����Ϊ$\frac{1}{2{t}_{2}}$mol/��L•min����
��5����t3ʱ�̣�������ת����Ϊ75%��
13��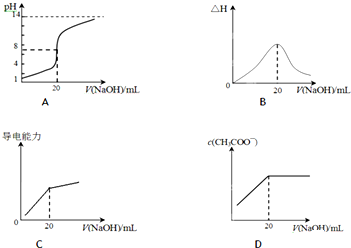 �����£�ijˮ��Һ��ֻ���������������ӣ�Na+��A-��H+��OH-��������ش��������⣮
�����£�ijˮ��Һ��ֻ���������������ӣ�Na+��A-��H+��OH-��������ش��������⣮
��1������0.2mol/L��HA��Һ��0.2mol/L��NaOH��Һ�������Ϻ���Һ��pH��7��������Һ�и�������Ũ���ɴ�С��˳���ǣ�c��Na+����c��A-����c��OH-����c��H+����
��2����HAΪ���ᣬ����������Һ
A�飺��0.4mol/L��HA��Һ��0.2mol/L��NaOH��Һ�������Ϻ���Һ��pH��7��
B�飺0.1mol/L��HA��Һ
��A����ҺpH��7��ԭ��Ũ��ʱ����HA�������A-����ˮ�⣮
��������Һ��c��A-���Ĵ�С��A�飾B�飨�������������=��������Һ��ˮ�ĵ���̶�A�飾B�飨�������������=������
��3����HAΪǿ�ᣬ����pH=2HA V1mL��pH=12Ba��OH��2 V2 mL��Ϻ�õ�pH=11����Һ����$\frac{{V}_{1}}{{V}_{2}}$=$\frac{9}{11}$
������Ũ��Ϊ0.1mol•L-1�����ֵ������Һ��NaHSO4����NaHCO3����NaCl����CH3COONa����NaOH
��1����������Һ��pH��С�����˳���Ǣ٣��ۣ��ܣ��ڣ��ݣ����ţ���
��2��������������Һ�зֱ����Al2��SO4��3��Һ���ܲ���������ɫ��ζ������Ǣڣ����ţ��������ӷ�Ӧ����ʽΪ��3HCO3-+Al3+=Al��OH��3��+3CO2����
����֪��
��1����Ũ�Ⱦ�Ϊ0.01mol/L��CH3COONa��NaClO��Na2CO3�Ļ����Һ�У���μ���0.01mol/L HCl������ϵ��������ӷ�Ӧ���Ⱥ�˳��ΪCO32-��ClO-��CH3COO-������������ӷ��ţ�
��2��25��ʱ����20mL 0.1mol/L CH3COOH��Һ����εμ�0.1mol/L NaOH��Һ����ϵ�и���������NaOH��Һ�ļ�����仯��ͼ����ȷ����D��
 �����£�ijˮ��Һ��ֻ���������������ӣ�Na+��A-��H+��OH-��������ش��������⣮
�����£�ijˮ��Һ��ֻ���������������ӣ�Na+��A-��H+��OH-��������ش��������⣮��1������0.2mol/L��HA��Һ��0.2mol/L��NaOH��Һ�������Ϻ���Һ��pH��7��������Һ�и�������Ũ���ɴ�С��˳���ǣ�c��Na+����c��A-����c��OH-����c��H+����
��2����HAΪ���ᣬ����������Һ
A�飺��0.4mol/L��HA��Һ��0.2mol/L��NaOH��Һ�������Ϻ���Һ��pH��7��
B�飺0.1mol/L��HA��Һ
��A����ҺpH��7��ԭ��Ũ��ʱ����HA�������A-����ˮ�⣮
��������Һ��c��A-���Ĵ�С��A�飾B�飨�������������=��������Һ��ˮ�ĵ���̶�A�飾B�飨�������������=������
��3����HAΪǿ�ᣬ����pH=2HA V1mL��pH=12Ba��OH��2 V2 mL��Ϻ�õ�pH=11����Һ����$\frac{{V}_{1}}{{V}_{2}}$=$\frac{9}{11}$
������Ũ��Ϊ0.1mol•L-1�����ֵ������Һ��NaHSO4����NaHCO3����NaCl����CH3COONa����NaOH
��1����������Һ��pH��С�����˳���Ǣ٣��ۣ��ܣ��ڣ��ݣ����ţ���
��2��������������Һ�зֱ����Al2��SO4��3��Һ���ܲ���������ɫ��ζ������Ǣڣ����ţ��������ӷ�Ӧ����ʽΪ��3HCO3-+Al3+=Al��OH��3��+3CO2����
����֪��
| �� | ���볣����Ka�� | �� | ���볣����Ka�� |
| CH3COOH | 1.8��10-5 | HCN | 5��10-10 |
| H2CO3 | Ka1=4.2��10-7 | HClO | 3��10-8 |
| Ka2=5.6��10-11 |
��2��25��ʱ����20mL 0.1mol/L CH3COOH��Һ����εμ�0.1mol/L NaOH��Һ����ϵ�и���������NaOH��Һ�ļ�����仯��ͼ����ȷ����D��

 ��
��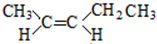 ��
��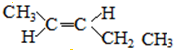 ��
�� ��
��