��Ŀ����
8��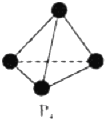 VA��ĵ����ס��飨As����Ԫ���ڻ������г����ֳ���������̬����VA��Ԫ�صĻ��������о������ش��������⣺
VA��ĵ����ס��飨As����Ԫ���ڻ������г����ֳ���������̬����VA��Ԫ�صĻ��������о������ش��������⣺��1�����ʵij�����ʽ�а��ס����ȣ����Ľṹ��ͼ��ʾ������Ϊ60�㣬Pԭ�Ӳ��õĹ���ӻ���ʽ��sp3��������ѧ֪ʶ�������жϰ������ڷ��Ӿ��壮
��2��N2�ĽṹʽΪN��N�����ЦҼ���м�����֮��Ϊ1��2��
��3��Asԭ������Ϊ33�������۲���ӵĹ����ʾʽΪ
 ��N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��P��As��
��N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��P��As����4��NH3��PH3��AsH3�ۡ��е��ɸߵ��͵�˳��ΪNH3��AsH3��PH3��ԭ���Ƿ��Ӿ�����۷е�������Է�����������������������д�������������۷е���ߣ�
��5��������һ�־���ǿ�����Ե�һԪǿ�ᣬNO${\;}_{3}^{-}$���ӵ����幹��Ϊƽ�������Σ���д��һ����NO${\;}_{3}^{-}$��Ϊ�ȵ����������ڷǼ��Է��ӵ����Ļ�ѧʽSO3��
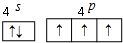
���� ��1������������������ṹ���ĸ�Pԭ��λ���������嶥���ϣ�ÿ��Pԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Pԭ���ӻ���ʽ�����Ĺ������Ƿ��ӣ�
��2�����������е�ԭ��֮�����3�����õ��Ӷԣ�ÿ�����������к���1���Ҽ���2���м���
��3��As��ԭ��������33�������������Ǽ۲���ӣ�ͬһ����Ԫ�ص�һ����������ԭ�������������С��
��4���⻯�ﶼ�Ƿ��Ӿ��壬���Ӿ�����۷е�������Է������������ȣ�������������⻯���۷е�ϸߣ�
��5����������Ӽ۲���ӶԸ�����3�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж���������ӿռ乹�ͣ���������ӵļ۵�������24����NO3-��Ϊ�ȵ����������ڷǼ��Է�������������
��� �⣺��1������������������ṹ���ĸ�Pԭ��λ���������嶥���ϣ����Լ�����60�㣻ÿ��Pԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ�������֪Pԭ���ӻ���ʽΪsp3�����Ĺ������Ƿ��ӣ��������Ƿ��Ӿ��壬�ʴ�Ϊ��60�㣻sp3�����ӣ�
��2�����������е�ԭ��֮�����3�����õ��Ӷԣ���ṹʽΪN��N��ÿ�����������к���1���Ҽ���2���м������ԦҼ���м�����֮��Ϊ1��2���ʴ�Ϊ��N��N��1��2��
��3��As��ԭ��������33�������������Ǽ۲���ӣ��۵��ӹ����ʾʽΪ����۲���ӵĹ����ʾʽΪ�� ��N��P��As����ͬһ���壬��ԭ��������������ԭ������Խ������������Խ����ʧȥ������������ԽС������������Ԫ�صĵ�һ�����ܴ�С˳����N��P��As��
��N��P��As����ͬһ���壬��ԭ��������������ԭ������Խ������������Խ����ʧȥ������������ԽС������������Ԫ�صĵ�һ�����ܴ�С˳����N��P��As��
�ʴ�Ϊ��33�� ��N��P��As��
��N��P��As��
��4���⻯�ﶼ�Ƿ��Ӿ��壬���Ӿ�����۷е�������Է������������ȣ�������������⻯���۷е�ϸߣ����������к�������������۷е���ߣ��۷е�ߵ�˳����NH3��AsH3��PH3��
�ʴ�Ϊ��NH3��AsH3��PH3�����Ӿ�����۷е�������Է�����������������������д�������������۷е���ߣ�
��5����������Ӽ۲���ӶԸ�����3�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ�������֪��������ӿռ乹��Ϊƽ�������Σ���������ӵļ۵�������24����NO3-��Ϊ�ȵ����������ڷǼ��Է�����SO3��
�ʴ�Ϊ��ƽ�������Σ�SO3��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰ԭ���ӻ���ʽ�жϡ����ռ乹���жϡ�ԭ�Ӻ�������Ų����ȵ������֪ʶ�㣬��ȷ�۲���ӶԻ������ۡ�����ԭ�����ȵ��������۵�֪ʶ�㼴�ɽ���ѵ��Ǽ���۲���ӶԸ�����

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�| A�� | ����������ϡ�����У�FeS+2H+�TFe2++H2S�� | |
| B�� | NH4NCO3���ڹ�����NaOH��Һ�У�HCO3-+OH+�TCO32-+H2O | |
| C�� | ����CO2ͨ�뱽������Һ�У�C6H5O-+CO2+H2O��C6H5OH+CO32- | |
| D�� | ����ˮ�еμ�����������Һ��OH-+H+�TH2O |
| A�� | ��������ά�ػ�Ϊͬ���칹�� | |
| B�� | ��ϩ�ܺ���ˮ�����Ը��������Һ�����ӳɷ�Ӧʹ֮��ɫ | |
| C�� | ���͡����ͺ�ֲ���Ͷ���̼�⻯�����ȫȼ��ֻ����CO2��H2O | |
| D�� | ���ۡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ |
| A�� | ��Mg��HCO3��2��Һ�м��������NaOH��Һ��Mg2++2HCO3-+2OH-=MgCO3��+2H2O+CO32- | |
| B�� | Ư����Һ��ͨ�����CO2���壺Ca2++2ClO-+CO2+H2O=CaCO3��+2HClO | |
| C�� | ��NH4Al��SO4��2��Һ���������Ba��OH��2��Һǡ��ʹSO42-��Ӧ��ȫ�� 2Ba2++4OH-+Al3++2SO42-=2BaSO4��+AlO2-+2H2O | |
| D�� | FeBr2��Һ��ͨ�������Cl2��2Fe2++4Br-+3Cl2=2Fe3++2Br2+6Cl- |
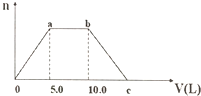 ��Ba��OH��2��NaOH�Ļ����Һ��ͨ��������CO2���壬���ɳ��������ʵ�����n����ͨ��CO2����������V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
��Ba��OH��2��NaOH�Ļ����Һ��ͨ��������CO2���壬���ɳ��������ʵ�����n����ͨ��CO2����������V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | a��֮ǰ�ķ�Ӧ��ѧ����ʽΪ��CO2+Ba��OH��2=BaCO3��+H2O | |
| B�� | a��b֮��ķ�Ӧ���ӷ���ʽΪ��CO2+2OH-=CO${\;}_{3}^{2-}$+H2O | |
| C�� | c��CO2�����ӦΪ15.0L | |
| D�� | ԭ�����Һ��Ba��OH��2��NaOH��Ũ��֮��Ϊ1��1 |
| A�� | K+��CH3COOH��CO32-��NO3- | B�� | Fe3+��Na+��SCN-��Cl- | ||
| C�� | Ba2+��Na+��OH-��HCO3- | D�� | H+��K+��Fe3+��NO3- |
| A�� | 1Ħ��H2O����������Ϊ12NA | |
| B�� | 2����������ԭ����ΪNA | |
| C�� | 0.5Ħ�����������������ᷴӦת�Ƶ�����Ϊ1.5NA | |
| D�� | ��״���£�1��ˮ����������Ϊ$\frac{1}{22.4}$ NA |