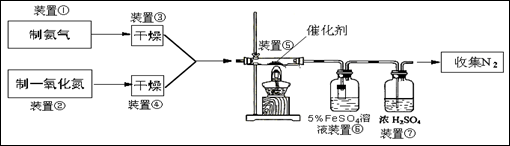
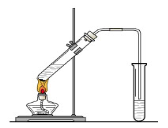
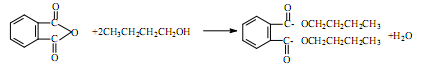��Ŀ����
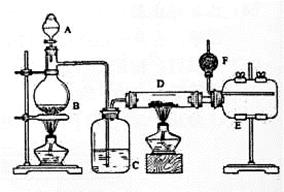
��ȸʯ����Ҫ�ɷ�ΪCu2(OH)2CO3����������Fe��Si�Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O�IJ������£�
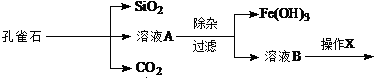
 Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
(1)�����ӡ�ʱ��ͨ������H2O2��Fe2��������Fe3�����ټ���CuO���塣���м���CuO������
���������ҺpH�ķ�ΧΪ ��
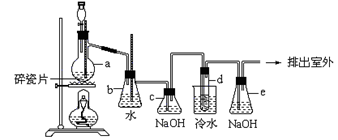
(2)����X���� ����Ũ������ȴ�ᾧ�����˺�ϴ�ӵȡ��ڽ��иò���ʱ����Ҫ����ҺB��
�ʵ��ữĿ�� ��
(3)������ҺB����μ���NaOH��Һ���պó��ֳ���ʱ����д�����ڵ��������ʵij����ܽ�ƽ�ⷽ��ʽΪ
(4) Ϊȷ�ⶨ��ҺA�к���Fe2�������ʵ���Ũ�ȣ�ʵ�����£�
��ȡ��25.00mL��ҺA�����Ƴ�250 mL ��Һ��
�ڵζ���ȷ��ȡ25.00ml������Һ����ƿ�У���0.20mol/LKMnO4��Һ
װ�� ����¼���ݡ��ظ��ζ�2�Ρ�ƽ������KMnO4��ҺV mL��
(��Ӧʽ�� 5Fe2+ + MnO4�� +10 H+ = 5Fe3+ + Mn2+ + 5H2O)
�� ������ҺA��Fe2+�����ʵ���Ũ��= mol/L ��ֻ�г���ʽ���������㣩��
|

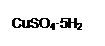 Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�| ���� | pH (��ʼ����) | pH(��ȫ����) |
| Fe(OH)3 | 1.9 | 3.2 |
| Fe(OH)2 | 7.0 | 9.0 |
| Cu(OH)2 | 4.7 | 6.7 |
���������ҺpH�ķ�ΧΪ ��
(2)����X���� ����Ũ������ȴ�ᾧ�����˺�ϴ�ӵȡ��ڽ��иò���ʱ����Ҫ����ҺB��
�ʵ��ữĿ�� ��
(3)������ҺB����μ���NaOH��Һ���պó��ֳ���ʱ����д�����ڵ��������ʵij����ܽ�ƽ�ⷽ��ʽΪ
(4) Ϊȷ�ⶨ��ҺA�к���Fe2�������ʵ���Ũ�ȣ�ʵ�����£�
��ȡ��25.00mL��ҺA�����Ƴ�250 mL ��Һ��
�ڵζ���ȷ��ȡ25.00ml������Һ����ƿ�У���0.20mol/LKMnO4��Һ
װ�� ����¼���ݡ��ظ��ζ�2�Ρ�ƽ������KMnO4��ҺV mL��
(��Ӧʽ�� 5Fe2+ + MnO4�� +10 H+ = 5Fe3+ + Mn2+ + 5H2O)
�� ������ҺA��Fe2+�����ʵ���Ũ��= mol/L ��ֻ�г���ʽ���������㣩��
�� ������Һ��pH���ٽ�Fe3��ˮ������Fe(OH)3������3�֣���3.2��4.7��2�֣�
�� ����Cu2����ˮ�� ��3�֣� (3) Cu(OH)2(s) Cu 2+(aq) + 2OH -(aq) ��2�֣�
Cu 2+(aq) + 2OH -(aq) ��2�֣�
(4)����ʽ�ζ��ܣ�2�֣� �� ��0.20��V��10-3��5 /25 ��10-3 ��2�֣�
��0.20��V��10-3��5 /25 ��10-3 ��2�֣�
�� ����Cu2����ˮ�� ��3�֣� (3) Cu(OH)2(s)
 Cu 2+(aq) + 2OH -(aq) ��2�֣�
Cu 2+(aq) + 2OH -(aq) ��2�֣�(4)����ʽ�ζ��ܣ�2�֣� ��
 ��0.20��V��10-3��5 /25 ��10-3 ��2�֣�
��0.20��V��10-3��5 /25 ��10-3 ��2�֣������������1��Ҫ��ȥ��Fe3+���ӣ�����Ҫ������Һ��pHֵ���һ����������µ����ʣ���������ͭ�����þ���������Һ��pH���ٽ�Fe3��ˮ������Fe(OH)3������������ͭ��ʼ����ʱ��pHֵ��4.7������������ȫ����ʱ��pHֵʱ3.2������pH�ķ�Χ��3.2��4.7��
��2������ͭ��������Һ��Ҳˮ�⣬�����ٴ��ữ��Ŀ��������Cu2����ˮ�⡣
��3��������ͭ�ij����ܽ�ƽ�ⷽ��ʽ��Cu(OH)2(s)
 Cu 2+(aq) + 2OH -(aq)��
Cu 2+(aq) + 2OH -(aq)����4�������ڸ�����ؾ���ǿ�����ԣ���ֻ������ʽ�ζ���ʢװ���������Һ��
�۸��ݷ�Ӧ�ķ���ʽ��֪��1mol�������������5mol�������ӣ����Ը������ĵĸ�����ص����ʵ�����֪����ҺA��Fe2+�����ʵ���Ũ��
 ��0.20��V��10-3��5 /25 ��10-3mol/L��
��0.20��V��10-3��5 /25 ��10-3mol/L�������������Ǹ߿��еij������ͣ��Ѷȴ��ۺ���ǿ����ѧ����Ҫ��ߡ�������ע�ضԻ���֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���淶�Ͻ���ʵ����������Լ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ����������������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
�����Ŀ