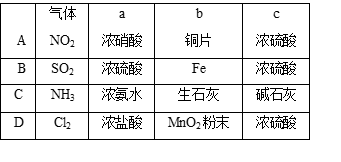
��Ŀ����
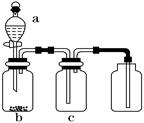
1,2-��������������Ϳ����������Ӽ�,������������ɫҺ��,�ܶ���2.18 g/cm3,�е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1,2-�������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ��ˮ(���渲������ˮ)������д���пհף�
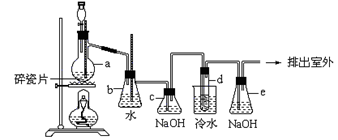
��1����ƿa�з��������Ҵ�����ˮ��Ӧ������ȥ��Ӧ����Ӧ�¶���170�棬���Ҹ÷�ӦҪ���¶�Ѹ�����ߵ�170�棬��������������Ӧ����д���Ҵ�������ȥ��Ӧ�ķ���ʽ ��
��2��д���Ʊ�1,2-��������Ļ�ѧ����ʽ�� ��
��3����ȫƿb���Է�ֹ����,�����Լ��ʵ�����ʱ�Թ�d�Ƿ�����������ش�������ʱƿb�е����� ��
��4������c��NaOH��Һ�������ǣ� ��
��5��ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û������,�Է�������ܵ�ԭ��_______________��

��1����ƿa�з��������Ҵ�����ˮ��Ӧ������ȥ��Ӧ����Ӧ�¶���170�棬���Ҹ÷�ӦҪ���¶�Ѹ�����ߵ�170�棬��������������Ӧ����д���Ҵ�������ȥ��Ӧ�ķ���ʽ ��
��2��д���Ʊ�1,2-��������Ļ�ѧ����ʽ�� ��
��3����ȫƿb���Է�ֹ����,�����Լ��ʵ�����ʱ�Թ�d�Ƿ�����������ش�������ʱƿb�е����� ��
��4������c��NaOH��Һ�������ǣ� ��
��5��ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û������,�Է�������ܵ�ԭ��_______________��
��10�֣�ÿ��2�֣���1��CH3CH2OH CH2��CH2����H2O��
CH2��CH2����H2O��
��2�� CH2��CH2��Br2 �� CH2BrCH2Br��
��3��b��ˮ����½����������е�ˮ������������������
��4����ȥ��ϩ�д��������������������̼����������
��5������ϩ����(��ͨ��Һ��)�ٶȹ����ʵ�������,��ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棻
 CH2��CH2����H2O��
CH2��CH2����H2O����2�� CH2��CH2��Br2 �� CH2BrCH2Br��
��3��b��ˮ����½����������е�ˮ������������������
��4����ȥ��ϩ�д��������������������̼����������
��5������ϩ����(��ͨ��Һ��)�ٶȹ����ʵ�������,��ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棻
�����������1���Ҵ�������ȥ��Ӧ������ϩ�����Է�Ӧ�Ļ�ѧ����ʽ��CH3CH2OH
 CH2��CH2����H2O��
CH2��CH2����H2O����2����ϩ����ˮ�����ӳɷ�Ӧ��������1��2���������飬��Ӧ�Ļ�ѧ����ʽ��CH2��CH2��Br2 �� CH2BrCH2Br��
��3�����d�з�����������b��ѹǿ��Ȼ���Ӷ�b��ˮ����½����������е�ˮ������������������
��4������Ũ�������ǿ�����ԣ��ܰ��Ҵ���������SO2��CO2���Ӷ�����ʵ�飬��������c��NaOH��Һ�������dz�ȥ��ϩ�д��������������������̼����������
��5��һ����������ϩ����(��ͨ��Һ��)�ٶȹ��죬���²�����ϩû����ȫ�����ա����ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棬�и���Ӧ������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���ض�ʵ����ƺͶ��ֲ�������������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ
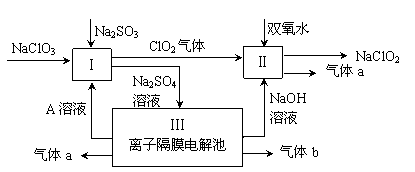
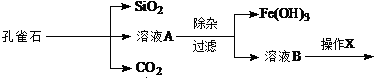
 Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�