��Ŀ����
�ܻ���DBP���ڱ����������������ҪӦ����PVC�Ⱥϳɲ�����������������Ӧԭ��Ϊ��
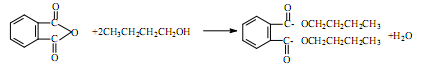
ʵ�鲽�����£�
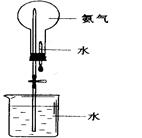
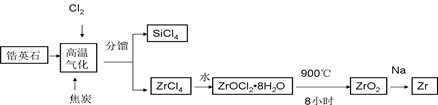
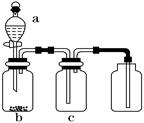
����1��������ƿ�з���14.8g�ڱ�����������25mL��������4��Ũ���ᣬ����������b����Ӧװ����ͼ����
����2�������������ڱ���������������ʧ�����������ڡ�
����3����������һ���̶�ʱ��������150��
����4����ȴ��������ƿ�е�Һ�嵹���©���У��ñ���ʳ��ˮ��5%̼����ϴ�ӡ�
����5����ѹ�����ռ�200~210��2666Pa��֣�����DBP��Ʒ
��1��Ũ��������� �������������� ��
��2����Ӧ������������������Ŀ���� ��
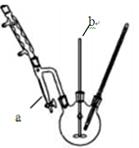
��3��ͼ������a�������Ƿ�ˮ�����Է������������� ��
����3��ȷ���д��������ɵ������� ��
��4��̼������Һϴ�ӵ�Ŀ���� ��
�ü�ѹ�����Ŀ���� ��
��5��д����������135�������ѵķ�Ӧ����ʽ ��
д��DBP������������Һ��ˮ��ķ���ʽ ��
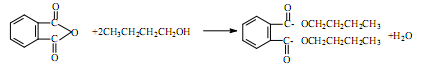
ʵ�鲽�����£�
����1��������ƿ�з���14.8g�ڱ�����������25mL��������4��Ũ���ᣬ����������b����Ӧװ����ͼ����
����2�������������ڱ���������������ʧ�����������ڡ�
����3����������һ���̶�ʱ��������150��
����4����ȴ��������ƿ�е�Һ�嵹���©���У��ñ���ʳ��ˮ��5%̼����ϴ�ӡ�
����5����ѹ�����ռ�200~210��2666Pa��֣�����DBP��Ʒ
��1��Ũ��������� �������������� ��
��2����Ӧ������������������Ŀ���� ��

��3��ͼ������a�������Ƿ�ˮ�����Է������������� ��
����3��ȷ���д��������ɵ������� ��
��4��̼������Һϴ�ӵ�Ŀ���� ��
�ü�ѹ�����Ŀ���� ��
��5��д����������135�������ѵķ�Ӧ����ʽ ��
д��DBP������������Һ��ˮ��ķ���ʽ ��
����12�֣�
��1����������ˮ����2�֣���1�� ʹ��Ӧ���ֻ��1��
��2�������������ĺ������ɴ�ʹ��Ӧ�����ƶ��������ڱ�����������ת����1��
��3����ʱ�����������Ӧ���ɵ�ˮ����ʹ��Ӧ�����ƶ���1��
��ˮ�����д�����ˮ����1��
��4����̼���Ƴ�ȥ���еĴ����1��
��ѹ����ɽ����л���ķе㣬���Է�ֹ�л�����ˮ̼������߲���Ĵ��ȡ�1��
��5��2CH3(CH2)2CH2OH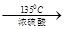 = CH3(CH2)3O(CH2)3CH3+H2O
= CH3(CH2)3O(CH2)3CH3+H2O
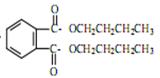 +2NaOH
+2NaOH  2CH3(CH2)2CH2OH+2H2O+
2CH3(CH2)2CH2OH+2H2O+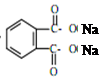
����ʽ��2�֣�������д������1��
��1����������ˮ����2�֣���1�� ʹ��Ӧ���ֻ��1��
��2�������������ĺ������ɴ�ʹ��Ӧ�����ƶ��������ڱ�����������ת����1��
��3����ʱ�����������Ӧ���ɵ�ˮ����ʹ��Ӧ�����ƶ���1��
��ˮ�����д�����ˮ����1��
��4����̼���Ƴ�ȥ���еĴ����1��
��ѹ����ɽ����л���ķе㣬���Է�ֹ�л�����ˮ̼������߲���Ĵ��ȡ�1��
��5��2CH3(CH2)2CH2OH
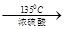 = CH3(CH2)3O(CH2)3CH3+H2O
= CH3(CH2)3O(CH2)3CH3+H2O 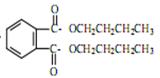 +2NaOH
+2NaOH  2CH3(CH2)2CH2OH+2H2O+
2CH3(CH2)2CH2OH+2H2O+
����ʽ��2�֣�������д������1��
�����������1������������Ϣ�ã�Ũ���������Ϊ��������ˮ����������������Ϊʹ��Ӧ���ֻ�ϡ�
��2����Ӧ������������������Ŀ���������������ĺ������ɴ�ʹ��Ӧ�����ƶ��������ڱ�����������ת���ʡ�
��3����ˮ����������ʱ�����������Ӧ���ɵ�ˮ����ʹ��Ӧ�����ƶ���ȷ���д��������ɵ������Ƿ�ˮ�����д�����ˮ���ɡ�
��4��̼������Һϴ�ӵ�Ŀ������̼���Ƴ�ȥ���еĴ����ᡣ�ü�ѹ�����Ŀ���Ǽ�ѹ����ɽ����л���ķе㣬���Է�ֹ�л�����ˮ̼������߲���Ĵ��ȡ�
��5������������Ϣ�ã���������135�������ѵķ�Ӧ����ʽΪ2CH3(CH2)2CH2OH
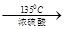 = CH3(CH2)3O(CH2)3CH3+H2O��DBP������������Һ��ˮ��ķ���ʽΪ
= CH3(CH2)3O(CH2)3CH3+H2O��DBP������������Һ��ˮ��ķ���ʽΪ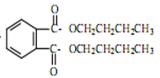 +2NaOH
+2NaOH 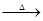 2CH3(CH2)2CH2OH+2H2O+
2CH3(CH2)2CH2OH+2H2O+ ��
�����������⿼������ܻ�����Ӧ�ú��л���Ӧ�����֪ʶ����Ŀ�Ѷȴ����ú����е���Ϣ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
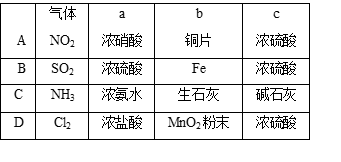
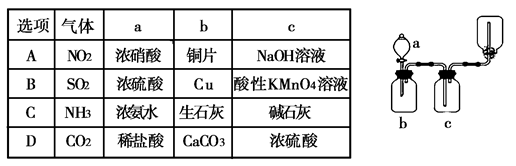
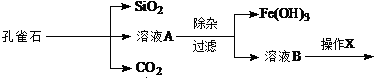
 Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�