��Ŀ����
����Ŀ�����Ȼ���(IC13)��ҩ��ϳ�����;�dz��㷺����֪ICl3�۵�33�棬�е�73�棬����ʪ�ԣ���ˮ��ˮ�⡣ijС��ͬѧ������װ����ȡICl3(���ּгֺͼ���װ��ʡ��)��
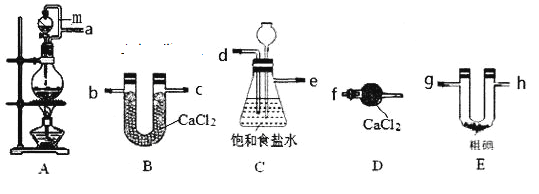
(1)���������������ӽӿ�˳��Ϊa��__________________________________________��װ��A�е���m��������____________________________________________��
(2)װ��C���ڳ��ӣ�ͬʱ��Ϊ��ȫƿ���ܼ��ʵ�����ʱ����װ���Ƿ�������������������C�е�����Ϊ____________________________________________________��
(3)�����뵥�ʵ������¶��Ե���70���·�Ӧ����װ��E���˵ļ��ȷ�ʽΪ____________��װ��E�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
(4)��װ�ô��ڵ�����ȱ����_____________________________________��
(5)�ֵ���Ʊ�����Ϊ��

����Y�õ��IJ����������ձ���____________������Z������Ϊ__________��
���𰸡�a��d��e��b��c��g��h��f�����ӿ�b��c��g��h�ɻ����� ʹŨ����˳������ ��ƿ��Һ���½�������©����Һ������ ˮԡ���� 3Cl2+I2 ![]() 2ICl3 ȱ��β������װ�� ��Һ©�� ����
2ICl3 ȱ��β������װ�� ��Һ©�� ����
��������
(1)װ������˳��Ϊ��ȡ����װ�á���������װ�á���������װ�á�������Ӧװ�á���ֹ����װ�ã�
(2)װ��C����ȫƿ�����ʵ�����ʱB���Ƿ���������B��������ʱ��C��ѹǿ��������ѹǿ��Һ�����÷���ʵ������
(3)ˮԡ���¶Ȳ�����100������ˮԡ�����ܼ����Ƽ����¶ȣ�ʹ�����������Ⱦ��ȣ�������֪���ʡ��Ʊ����ʣ���Ϸ�Ӧ������������Ӧ��Ӧ������д��Ӧ����ʽ��
(4)�������ж������壬Ҫ��β������װ�ã�
(5)����Y����ȡ��ʹ�÷�Һ©�����ձ�������Z������
(1)��װ��A����Ũ������MnO2��ϼ�����ȡCl2��Ũ�����ӷ���ʹ��Ӧ��ȡ�������к����Ȼ��⡢ˮ���������ʣ�ͨ��װ��C��ȥHCl���ʣ���ͨ��װ��B����õ����������������Ȼ����װ��E��������ⵥ�ʷ�Ӧ�Ƶ�ICl3��Ϊ��ֹICl3���⣬��������ʢ�и������Dװ�ã���ֹ�����е�ˮ�������뵽��ȡEװ�����ʰ���������������װ�ýӿ�˳��Ϊa��d��e��b��c��g��h��f�����ӿ�b��c��g��h�ɻ���������װ��A�е���mʹ��Һ©���е�Һ������ѹǿһ�£�������Һ©���е�Ũ����Ϳ���˳�����£�
(2)װ��C���ǰ�ȫƿ�����ʵ�����ʱB���Ƿ�����������װ��B����������C������ѹǿ����Һ�����뵽����©���У�ʹ��ƿ��Һ���½�������©����Һ��������
(3)��ˮԡ�����ܼ����Ƽ��ȵ��¶ȣ�����ʹ��Ӧ�Թ����Ⱦ��ȣ����������뵥�ʵ������¶��Ե���70���·�Ӧ����Ӧ��ȡˮԡ���ȵķ�ʽ����װ��E��Cl2��I2�ڵ���70���¶��·�����Ӧ��3Cl2+I2 ![]() 2ICl3��
2ICl3��
(4)Cl2���ж����壬ʢ����ˮ�Ȼ��Ƶĸ����ֻ������ˮ�֣��������������������ͻ���ɴ�����Ⱦ��������ˮCaCl2���ɼ�ʯ�ң��Ϳ�������������ˮ�����������˴�����Ⱦ���ʸ�װ�õ�ȱ����ȱ��β������װ�ã�
(5)����Y�Ǽ���CCl4������I2�ӵ�ˮ����ȡ������������õ��IJ����������ձ��ͷ�Һ©�������������Ȼ�̼��Һ���������ܼ����Ը��ݶ��߷е㲻ͬ��������ķ����������ǡ�

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�