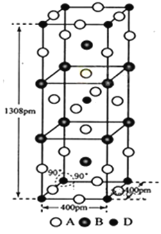
��Ŀ����
����Ŀ���л��ϳɵ���Ҫ�м���F��һ�ֺϳ�·�����£�
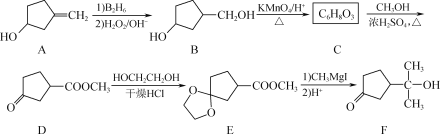
(1) F�к��������ŵ�������________��________��
(2) C�Ľṹ��ʽΪ________��
(3) ���������̿���D��E��������___________��
(4) GΪ��E��Է���������14��ͬϵ�H��G��Ϊͬ���칹���ҷ�������������
�� 1 mol H����2 mol NaHCO3��Ӧ��
�� H��ʹ������Ȼ�̼��Һ��ɫ��
�� H�����к���3�ֲ�ͬ��ѧ�������⡣
��H�Ľṹ��ʽΪ________(дһ��)��
(5) д����![]() ��CH3OH��CH3CH2MgIΪ��Ҫԭ���Ʊ�
��CH3OH��CH3CH2MgIΪ��Ҫԭ���Ʊ�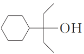 �ĺϳ�·��____________ (���Լ����л��ܼ����ã��ϳ�·������ͼʾ�����������)��
�ĺϳ�·��____________ (���Լ����л��ܼ����ã��ϳ�·������ͼʾ�����������)��
���𰸡��ǻ� �ʻ� ![]() �����ʻ�����ֹ����CH3MgI��Ӧ
�����ʻ�����ֹ����CH3MgI��Ӧ  ��
�� ��
��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
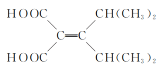
A�����ӳɷ�Ӧ����B��B������ط�Ӧ����C������C��Ӧ����D�Ľṹ�ͷ���ʽ���ӵõ�C�Ľṹ��ʽΪ![]() ��D���Ҷ�������ȡ����Ӧ����E��E��CH3MgI��Ӧ����H+��Ӧ�õ�F��
��D���Ҷ�������ȡ����Ӧ����E��E��CH3MgI��Ӧ����H+��Ӧ�õ�F��
(1)����F�Ľṹ��ʽ�õ�F�ĺ��������ŵ��������ǻ����ʻ����ʴ�Ϊ���ǻ����ʻ���
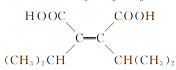
(2)����ǰ������õ�C�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)���������̿���D��E��E��F���ʻ������Ҷ�����Ӧ�������������ʻ���˵��D��E�������DZ����ʻ�����ֹ����CH3MgI��Ӧ���ʴ�Ϊ�������ʻ�����ֹ����CH3MgI��Ӧ��
(4)GΪ��E��Է���������14��ͬϵ���G�ķ���ʽΪC10H16O4��H��G��Ϊͬ���칹���ҷ���������������1 mol H����2 mol NaHCO3��Ӧ��˵������2mol�Ȼ����� H��ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼˫������ H�����к���3�ֲ�ͬ��ѧ�������⣻��H�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(5)��![]() ������������ͭ��Ӧ�����ữ�õ�
������������ͭ��Ӧ�����ữ�õ�![]() ��
��![]() �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ����![]() ��
��![]() ��״���Ũ������������·�Ӧ������(
��״���Ũ������������·�Ӧ������(![]() )��
)��![]() ��CH3CH2MgI��Ӧ����H+��Ӧ�õ�
��CH3CH2MgI��Ӧ����H+��Ӧ�õ�![]() ��������ͼΪ��
��������ͼΪ��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() ��
��

 ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ��I������(H2C2O4)���������������������ܹ�������Ӧ��
��ʵ��1����ͬѧ��8.00 mL 0.001 mol/L KMnO4��Һ��5.00 mL 0.01 mol/L H2C2O4��Һ��Ӧ���о���ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�졣�ı���������£�
��� | KMnO4��Һ /mL | H2C2O4��Һ /mL | 10%�������/mL | �¶�/�� | �������� |
�� | 8.00 | 5.00 | 3.00 | 20 | |
�� | 8.00 | 5.00 | 3.00 | 30 | |
�� | 8.00 | 5.00 | 1.00 | 20 | 2.00 mL����ˮ |
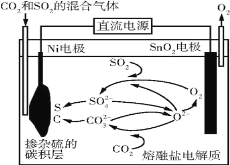
��1��д������(H2C2O4)����������Һ�����������·�Ӧ�����ӷ���ʽ________��
��2����������ʵ����Ŀ����̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��ʵ��2����ͬѧ���о������������������������·�Ӧ��Ӱ������ʱ����,���������Ը��������Һ��ʼһ��ʱ�䷴Ӧ���ʽ���,��Һ��ɫ������,�����ú�ͻȻ��ɫ,��Ӧ�������Լӿ졣
��3�������������,��ͬѧ��Ϊ�����������ط�Ӧ����,������Һ�¶�����,��Ӧ���ʼӿ졣��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�,����뻹������_______��Ӱ�졣
��4������ʵ��֤����IJ���,�������Ը��������Һ�Ͳ�����Һ��,����Ҫѡ����Լ����������____������ĸ����
a������� b��ˮ c���������� d��������
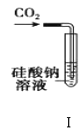
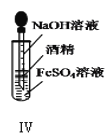
������ͼ��ʾ��װ�ý����к��ȵIJⶨʵ�飬�ֱ�ȡ![]() ��
��![]() ��Һ��
��Һ��![]() ���������ʵ�飬�ش��������⣺
���������ʵ�飬�ش��������⣺
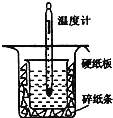
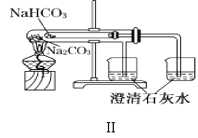
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________������֮�⣬װ���е�һ�����Դ�����__________��
��2��������Ϊ![]() ��NaOH��Һ��
��NaOH��Һ��![]() ��������Һ���ܶȶ���
��������Һ���ܶȶ���![]() ���кͺ�������Һ�ı�����
���кͺ�������Һ�ı�����![]() ��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ||
H2SO4 | NaOH | ƽ��ֵ | ||
1 | 26.2 | 26.0 | 26.1 | 29.5 |
2 | 27.0 | 27.4 | 27.2 | 32.3 |
3 | 25.9 | 25.9 | 25.9 | 29.2 |
4 | 26.4 | 26.2 | 26.3 | 29.8 |
��3������ʵ����ֵ�����![]() ��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨ![]() ��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�![]() ��Һ����ʢ�������С�ձ���
��Һ����ʢ�������С�ձ���
d����������ʵ������¶Ⱦ��������ƽ��ֵ
����Ŀ��������X����Y��Һ�У����ɳ������ʵ���n2������X�����ʵ���n1�Ĺ�ϵ��ͼ��ʾ������ͼ��ʾ�������
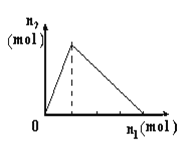
A | B | C | D | |
X | NaOH | AlCl3 | HCl | NaAlO2 |
Y | AlCl3 | NaOH | NaAlO2 | HCl |
A. A B. B C. C D. D