��Ŀ����
10��298Kʱ���ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��$?_{���¸�ѹ}^{����}$ 2NH3��g������H=-92.4kJ•mol-1���ڸ��¶��£�ȡ1molN2��3molH2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�����������С��92.4KJ����ԭ���Ǹ÷�Ӧ�ǿ��淴Ӧ��1molN2��3molH2��������ȫ��Ӧ�����Էų�����������С��92.4kJ������ �Ȼ�ѧ����ʽN2��g��+3H2��g��$?_{���¸�ѹ}^{����}$ 2NH3��g������H=-92.4kJ•mol-1�ĺ����ǣ���1mol������3mol������ȫ��Ӧ����2mol����ʱ����Ӧ����92.4KJ��
���÷�Ӧ�ǿ��淴Ӧ��ȡ1molN2��3molH2����һ�ܱ������У�������ȫ��Ӧ����2mol�������ݴ˷�����
��� �⣺�Ȼ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol��ʾ1mol������g����3mol������g������2mol������g����Ӧ������Ϊ92.4kJ�����ڸ÷�Ӧ�ǿ��淴Ӧ������1molN2��3molH2��������ȫ��Ӧ�����Էų�����������С��92.4kJ��
�ʴ�Ϊ���÷�Ӧ�ǿ��淴Ӧ��1molN2��3molH2��������ȫ��Ӧ�����Էų�����������С��92.4kJ��
���� ���鷴Ӧ�ȼ��㣬Ӧע���Ȼ�ѧ����ʽ�ĺ���ͷ�Ӧ�Ŀ����ԣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
1�������и�����������Һ�У�һ���ܴ���������������ǣ�������
| A�� | ��ˮϡ��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$�������Һ��K+��Na+��SO42-��AlO2- | |
| B�� | ������$\frac{{K}_{W}}{c��{H}^{+}��}$=0.1 mol/L����Һ��K+��Na+��SiO32-��NO3- | |
| C�� | ��Ƭ����������ݵ���Һ��Na+��NH4+��I-��NO3- | |
| D�� | NaHCO3��Һ��K+��Na+��SO42-��Al3+ |
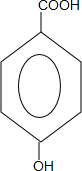
2�� ���������кȹ��ף�ƻ������һ����ƻ�����Ͷ��ɵľ��нⶾ����֬�����ʺ�ֹк������ҩЧ�Ľ���ʳƷ��ƻ���ᣨ�ṹ��ʽ��ͼ�����������ϵ���Ҫ�������ʣ��������˵������ȷ���ǣ�������
���������кȹ��ף�ƻ������һ����ƻ�����Ͷ��ɵľ��нⶾ����֬�����ʺ�ֹк������ҩЧ�Ľ���ʳƷ��ƻ���ᣨ�ṹ��ʽ��ͼ�����������ϵ���Ҫ�������ʣ��������˵������ȷ���ǣ�������
 ���������кȹ��ף�ƻ������һ����ƻ�����Ͷ��ɵľ��нⶾ����֬�����ʺ�ֹк������ҩЧ�Ľ���ʳƷ��ƻ���ᣨ�ṹ��ʽ��ͼ�����������ϵ���Ҫ�������ʣ��������˵������ȷ���ǣ�������
���������кȹ��ף�ƻ������һ����ƻ�����Ͷ��ɵľ��нⶾ����֬�����ʺ�ֹк������ҩЧ�Ľ���ʳƷ��ƻ���ᣨ�ṹ��ʽ��ͼ�����������ϵ���Ҫ�������ʣ��������˵������ȷ���ǣ�������| A�� | ƻ������һ���������ܷ���������Ӧ | |
| B�� | ƻ������һ���������ܷ�����������Ӧ | |
| C�� | 1molƻ������������Ʒ�Ӧ����1.5moL��H2 | |
| D�� | 1molƻ������Na2CO3��Һ��Ӧ��������2mol Na2CO3 |
19��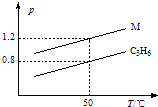 ͼ���������߷ֱ��ʾ1g C3H6��1g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ծ�ͼ���ж�M��������ǣ�������
ͼ���������߷ֱ��ʾ1g C3H6��1g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ծ�ͼ���ж�M��������ǣ�������
 ͼ���������߷ֱ��ʾ1g C3H6��1g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ծ�ͼ���ж�M��������ǣ�������
ͼ���������߷ֱ��ʾ1g C3H6��1g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ծ�ͼ���ж�M��������ǣ�������| A�� | SO2 | B�� | CO2 | C�� | C3H8 | D�� | CO��N2 |
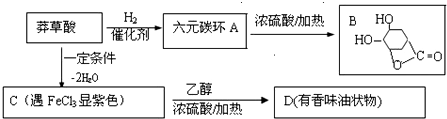
 ��
�� +CH3CH2OH$��_{��}^{Ũ����}$H20+
+CH3CH2OH$��_{��}^{Ũ����}$H20+ ��
�� �����������壨[CH3CH��OH��COO]2Fe•3H2O��Mr=288���dz��õIJ�������������������������̼���������ױ�������������������Ӧ�Ƶã�2CH3CH��OH��COOH+FeCO3��[CH3CH��OH��COO]2Fe+CO2��+H2O��
�����������壨[CH3CH��OH��COO]2Fe•3H2O��Mr=288���dz��õIJ�������������������������̼���������ױ�������������������Ӧ�Ƶã�2CH3CH��OH��COOH+FeCO3��[CH3CH��OH��COO]2Fe+CO2��+H2O��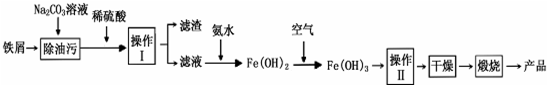
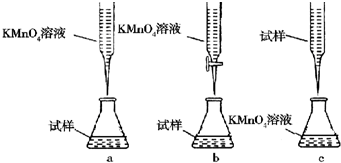
 ��
�� ��
��